Thalidomide has been found to have a negative effect on wound healing by reducing wound healing rate by 10-20% in laboratory studies (Tamilarasan et al, 2006). Angiogenesis is a vital event in wound healing and this process is also thought to be inhibited by thalidomide. Thus, these laboratory findings should be applied when developing management plans for hard-to-heal wounds in patients on thalidomide. Comorbidities, available resources and expertise are additional factors that affect hard-to-heal wound management in patients on thalidomide.
Here we describe a strategy for the successful management of progressively worsening, multiple lower limb wounds present for the last two years. The patient was a 75-year-old female on thalidomide for multiple myeloma. Treatment involved temporarily withholding thalidomide, using cadoxemer iodine, negative pressure wound therapy (NPWT), and performing a split-thickness skin graft (STSG).
Case
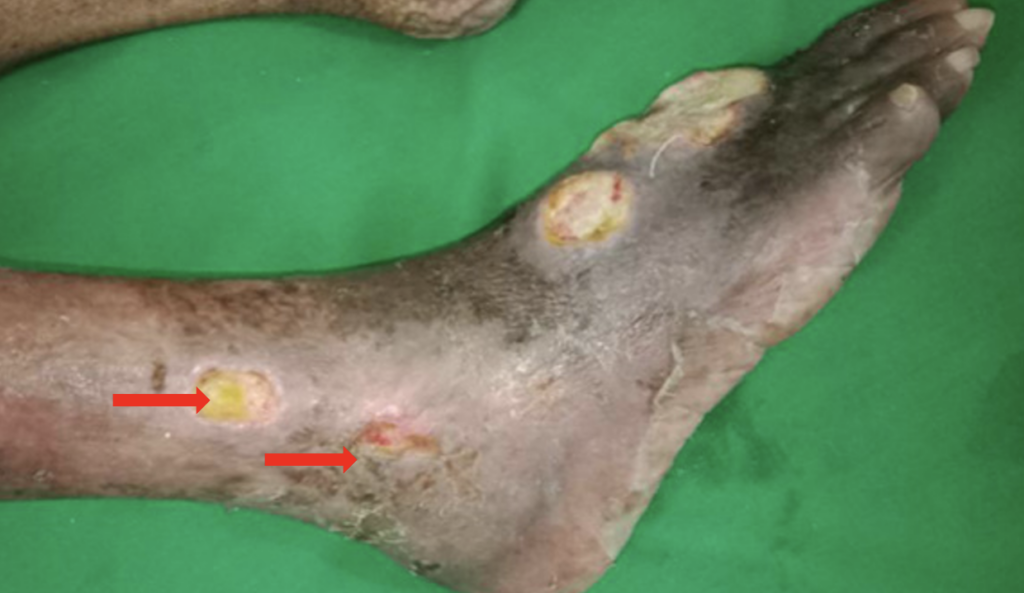
A 75-year-old female who did not have diabetes or occlusive arterial disease, but did have hypertension, dyslipidaemia, Vitamin D deficiency, hypothyroidism and multiple myeloma. She has completed six cycles of chemotherapy with bortezomib, cyclophosphamide and dexamethasone after the diagnosis of multiple myeloma two years ago and was currently on thalidomide 100mg at night. At the time of the multiple myeloma diagnosis, she had a 1cm sized wound over the left medial malleolus, which worsened with thalidomide therapy. At the time of presentation, she had two pus discharging wounds with an area of uncertain viability: a wound over the dorsum of the foot, measuring 6x5cm, and a wound over the medial malleolus, measuring 11 x 5cm (Figures 1A–1B). Subsequently, another three wounds developed over two weeks: one on the dorsum of the foot and two over the lateral malleolus, each measuring approximately 2cm (Figure 2).
The diagnosis of multiple myeloma was based on presence of monoclonal band in the gamma region, diminished albumin band, prominent alpha 1 and alpha 2 bands in serum electrophoresis, and immuneparesis favouring of multiple myeloma. Diagnosis was also based on paraprotein concentration of 10.94g/l, erythrocyte sedimentation rate (ESR) of 135mm/1st hour, urine bence jones protien negative, and an X-ray of the skull showing lytic lesions at the time of diagnosis.
On presentation to us, total white blood cell count was 11.71×103/µl , haemoglobin 10.3 g/dl, platelet count 622×103/µl, and ESR 120mm in the first hour. A wound swab was positive for Pseudomonas aeruginosa, which was sensitive for imipenem, meropenum, ciprofloxacin, cloxacillin, and cefexime. An X-ray of the foot and ankle did not show changes associated with osteomyelitis. Distal pulses in bilateral lower limbs were present.
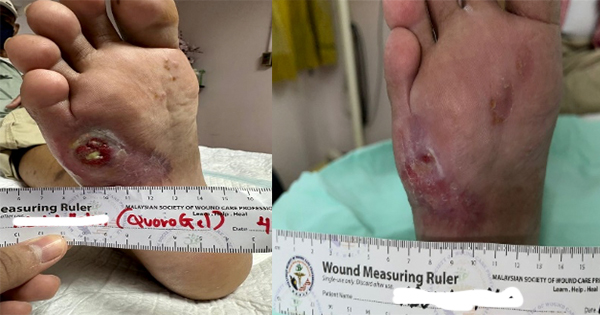
Initially, thalidomide was withheld with the oncologists’ consent, and the wounds were dressed with paraffin gauze and saline-soaked absorbent gauze every other day. The patient was started on ciprofloxacin based on the bacterial culture report. After the two weeks of withholding thalidomide, two wounds over the lateral malleolus healed completely while the wounds over the dorsum of the foot and medial malleolus left had demarcated well with slough (Figure 3A-3B)
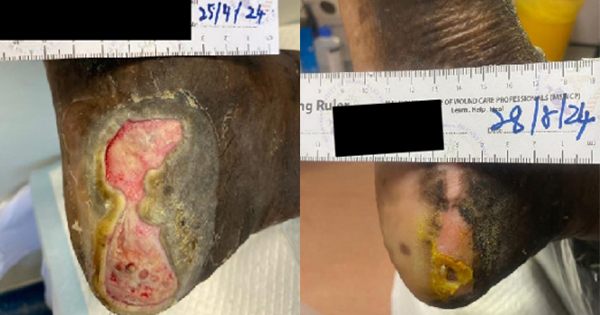
After bedside surgical debridement of the wounds over the lateral malleolus and dorsum of the foot, they were dressed with cadexomer iodine. This was intended to further deslough the remaining necrotic material adjacent to the Achillis tendon, posterior tibial vessels and around the tendons of the dorsum of the foot. Cadexomer iodine dressings were changed on every fourth to fifth day. At the end of the two weeks of dressing with cadexomer iodine, both wounds were devoid of slough, but the granulation tissues over the wound was not satisfactory enough to proceed with STSG (Figure 4A–4B).
With the aim of expediting the formation of healthy granulation tissue, on wounds over the dorsum of the foot and medial malleolus, 3, seven-day cycles of NPWT at -125mmHg subatmospheric pressure were applied using foam. At the end of the three weeks of NPWT, granulation tissue over the wounds was satisfactory for STSGs and there was no growth of organisms in the microbial cultures (Figure 5A–5B). STSGs were applied over the wounds under local anaesthesia, which were accepted and integrated well into the tissues at three weeks following grafting. Thus, it took a total of ten weeks for all the wounds to heal. During this period, the patient was carefully monitored with regular electrophoresis for worsening multiple myeloma. There was no evidence of worsening of the multiple myeloma during the10 weeks wound healing period. Thalidomide was restated after discussion with the oncologist. During the two years of follow-up, there was minor recurrence of a wound, which was managed conservatively with absorbent gauze dressings.
Physiotherapy to bilateral lower limbs was also implemented to help the patient recover from the period of debilitation due to these wounds.
Discussion
Multiple myeloma is characterised by immunosupression due to alteration in number and functionality of the immune populations (Diaz-Tejedor et al, 2021). Thalidomide is increasingly used to treat multiple myeloma and is known to inhibit angiogenesis (Moehler et al, 2008). Both immunosuppressive and anti-angiogenesis effects inhibit wound healing and therefore, management of chronic wounds in patients on thalidomide for multiple myeloma needs multiple strategies to suit the patient’s individual needs.
Anaemia, caused by multiple myeloma and old age, is an additional risk factor for poor wound healing identified in our patient.
Infected wounds with pus discharge and necrotic tissue need treatment to clear the infection and debridement of slough. Targeted antimicrobial therapies based on bacteria cultures and antimicrobial sensitivity tests are required to treat infection. Surgical debridement, the fastest and most cost-effective mode of debridement (Woo et al, 2015), was used to debride the wounds of our patient. Chemical debridement can be considered when the patient is not a surgical candidate or in wounds over delicate areas, such as over the bony prominences and around vital structures (Gray et al, 2011). Cadexomer iodine is found to be beneficial in chemical debridement of selected hard-to-heal wounds (Woo et al, 2021). Cadexomer iodine was used in our patient following surgical debridement to remove residual necrotic tissues over the blood vessels, tendons and in the margins of the wound.
Angiogenesis plays a crucial role in wound healing (Li et al, 2003). Angiogenesis begins at the time of tissue injury and persists throughout wound healing. Thalidomide arrests angiogenesis through multiple mechanisms (Tamilarasan et al, 2006). Withdrawal of agents that inhibit angiogenesis is essential for proliferation of tissues in the wound bed and periphery. Here, thalidomide withdrawal was carefully undertaken with multidisciplinary inputs from oncologists, and progression of disease during this period was assessed regularly with serum electrophoresis. Our patient did not show worsening of disease during the withholding of thalidomide, and this alone was effective in healing two of the wounds in our study.
On completion of debridement, there was a reasonable growth of granulation tissue in the wound bed for skin cover. NPWT is found to be effective in promoting granulation tissue growth, and NPWT was noted to expedite granulation tissue formation in the wound bed of our patient (Kunze et al, 2020).
Extra-long duration is unavoidable if spontaneous skin cover is allowed in large wounds. STSG takes only 14 days to integrate after successful implantation and should be the preferred option when working in a narrow window period.
In conclusion, early withdrawal of thalidomide, while monitoring the progression of multiple myeloma, targeted antimicrobial therapy, wound debridement with surgical or chemical methods, early NPWT and STSG, appears to be an effective strategy in the management of chronic wounds of patients on thalidomide therapy for multiple myeloma.