Implantable cardiac devices were first used in the 1950s, with the first implant pacemaker in a human carried out in 1958 by Dr. Senning (Haghjoo, 2018). Since then their use has continued to increase because of the effectiveness of these devices in increasing the life expectancy of patients with arrhythmias (Kotalczy K, 2020). There are three types of electronic cardiac implantable devices:
- Implantable cardiac devices (ICDs)
- Cardiac resyncronisation therapy devices (CRTDs)
- Permanent pacemaker (PPM).
A pacemaker system consists of one or two leads connected to a device that is implanted into a pocket formed just below the collarbone. Complications from these devices include, infection of the pocket device, haematoma of the pocket device, tamponade (fluid accumulation in the pericardial sac), pneumothorax (air leakin the space between the lung and chest wall), endocarditis (infection of the inner lining of the heart), phlebitis (inflammation of a vein near the surface of the skin) and perforation (Katsakuo et al, 2015). There are also infectious complications of the pacemaker, there is acute perforation, dislodge, infection, venous thrombosis and movement of the device.
In clinical practice, infection is often encountered with the incidence of using cardiac implants with major infection in within the first 12 months with pacemaker devices is 14.9%, with a CRT pacemaker it is 7.5%, infection using ICD is 16.4% and with a CRT-D is 61.2% (Wilkoff et al, 2020). Pichtchoulina and colleages report an incidence of CIED infection, caused by Staphylococcus aureus bacteria, is 37/ 61 patients (61%) and death in hospital occurred in 19/61 patients (31%; Pichtchoulin et al, 2021).
Infection is a state of invasion of the wound by the proliferation of microorganisms in local or systemic conditions depending on the condition or response of the host/client (Swanson, 2016). Infection resulting from of cardiac implantable devices need special attention because when ignored, there is a of risk septic shock, followed by cardiogenic shock. Cardiac implant infection is the result of the interaction between the device, host and microorganisms (Mulpuru et al, 2013)
Clinical manifestations
The clinical Aleka study, a guideline for cardiovascular implantable device (Hanafy et al, 2014), stated that symptoms can be as follows: fever (45%), shiver (43%), erythema (41%), swelling (38%), tenderness of the superficial pouch (28%), malaise (28%), erosion (21%) and warm (18%). Before infection, the initial signs of inflammation or an inflammatory response must be watched for. In the area of the superficial implant pocket these manifest as pain, swelling, redness and heat in the area around the superficial skin (Han and Roger, 2017).
Another possible results of infected implantable cardiac device, related infection after surgery, is surgical site infection (SSI). SSI a potential complication following any type of surgery (Sandy-Hodgetts, 2017). SSI is a complication postoperative discharge from acute care (Stryja et al, 2020). Infection occurs between 30 days and one year postoperatively, in a patient receiving an implant with either a superficial or deep tissue incision (ECDC, 2018). Evidence suggests after discharge 50% of SSI (Stryja et al, 2020) accounts for 18% from health care associated infections (Sueten et al, 2018). According to one study SSI results in 3% of patient dying (Minski, 2019).
Pathogenesis
Accurate diagnosis of SSI is difficult, as it may take several weeks to develop and many infection may not become apparent at patient discharge from the hospital (ECDC, 2023). Some cases result in readmission of a patient to the hospital with an infection 18% (Sueten et al, 2018).
SSI related to implantable cardiac devices fall in to the following board categories (Murpuru et al, 2013):
- Superficial infection: pocket or subcutaneous tissue infection. Erosion that can occur as a result of pressure from underlying implantable devices in the superficial tissue. Inflammatory phases such as pain, redness or heat, localised swelling and delayed healing leading to infection with purulent drainage from incision (Stryja et al, 2020).
- Deeper infection results in the spread of a superficial infection along the lead wire or secondary seeding of the implantable cardiac devices system. This can lead to endocarditis and bacteraemia. It can result in the body rejecting the foreign objects.
SSI of an implantable cardiac device is the result of interaction between the device, host and bacteria present. Device related factors that can affect adhesion by microorganisms included type of polymer coating, shape and irregularity of the surface.
Microorganism adherent to implanted cardiac devices may result in biofilm, bacteria encased in an extracellular polymeric substance matrix making them more resistant to antibiotic and the host defence system. This will be more of a problem when the infection reaches the lead wire, which is implanted in the myocardium, which can then lead to endocarditis and bacteraemia. (Sandoe et al, 2014).
Biofilm contains a matrix of proteins, fats and polysaccharides in the wound or on its surface, present in an estimated 78% chronic wounds (Malone et al, 2017). The most prevalent groups of bacteria being Pseudomonas aeruginosa and Staphylococus aureus (MC Nichole, 2016).
Negative pressure wound therapy (NPWT) is a standard treatment for open wounds, while there are few methods to treat closed surgical incision (Webster et al, 2012). NPWT facilitates the removal of wound exudate, which enhances blood circulation to wound bed, while keeping the wound environment moist. It also reduces bioburden and supports tissue granulation (Huang et al, 2014). NPWT is said to accelerated the healing a wide variety for traumatic, nontraumatic and chronic wounds (Agarwal et al, 2019). A systematic review from Sandy-Hodgetts et al (2015) reporting using NPWT for the prevention of surgical wound complications.
Wound treatment methods
The normal stages in the wound healing process are haemostasis or the coagulation process, inflammation, proliferation or regeneration and maturation or remodelling. This current concept put forward by experts in wound care known as moist wound healing (Schultz, 1993; McNichol, 2016). The key to moist wound healing or closed wound care is to prepare the wound bed.
In the process of wound care, management of infectioncan involve theuse of topical antimicrobials, such as silver, chlorhexidine, polyhexamethylen biguinide (PHMB), Octenidine, NaOCl, DACC, Iodine with a concentration of less than 1%, Charcoal, honey (Swanson, 2016) or systemic antibiotics. It also requires appropriate management of exudate, as well as the selection of hydrofoam dressing for the absorbtion of exudate.
NPWT can be used for exudate management. It works to suction wound fluids (Han and Roger, 2017). NPWT has a dressing connected to a pressure control device, with exudate collected a container or canester (Sagar et al, 2022).
The mechanism action of NPWT on open wounds cause a mechanical stress of the wound edge that alters tissue reperfusion, resulting in angiogenesis, stimulating blood flow and oxygenation, formation of granulation tissue and effective biochemical reduction of the fluid concentration wound healing impairing proteases (Apelqvist, 2017). The application of NPWT is associated with a lower risk of wound healing complication such as seroma, haematoma and infection and with an increasedrate of wound healing (Itani, 2015). The World Health Organisation (WHO) guidelines say for the prevention SSI use NPWT, this is a conditional recommendation with low evidence to support this indication (WHO, 2019).
Case report
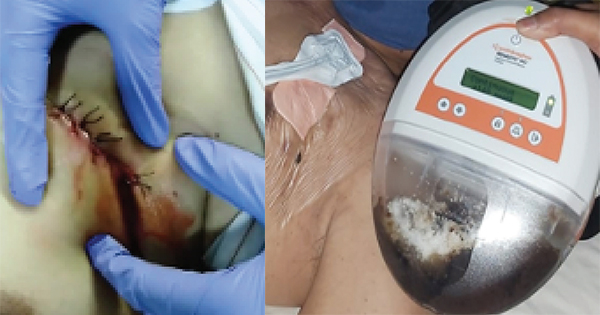
A case 68-year-old male, with a history of pain in the area where the CRT-D dual chamber heart implantable device was inserted 6 months ago. The pain has been present for 4 days, along with redness of skin and warmth around the left chest implant placement. After the sixth day, the patient complained of a yellowish wound fluid and is accompanied by fever. Swab culture of wound bed was performed and Staphylococus aureus bacteria was found to be present. The results of the chest X-ray showed no infiltrate, no pleural effusion, the cardiac implant generator had shifted in the left chest position, the ECG showed no changes.
The patients pain was still on a moderate scale visual analogue scale (VAS:1–10) was 5/10, there was necrotic tissue in the implant incision area and redness of the skin around the wound. The patient had a debridement was hospitalised to the semi-intensive care cardiovascular centre. After cleansing and debridement were performed to remove the necrotic tissue and exudate, while cleaning the pocket implantable using normal saline 0.9%. The incision site length was 7cm and the generator was left implanted.
The incusion was closed with sutures (primary intention healing method) and incision drainage site of 2cm by cutting sterile latex (conventional technique) was created.
Antibiotics were given intravenously, sulbactam ampicillin 2x 1.5gr (Hanafy et al, 2014). After 7 days there was no improvement, and a high volume of serosangounous exudate.
After seven days there was no change, so a second debridement was performed to remove the generator and lead wire. Then using wound care techniques the concept of moist wound healing was employed. The first step was cleaning debris and residual exudate with normal saline fluid 0.9%, then giving silver nano-spray as a topical antimicrobial. After which a transparent film dressing was applied for skin barrier protection. The length of the wound was 7cm, with an opened suture a hole 2cm and deep 1cm was made in the centre of wound bed for the granulofoam and to connect softport negative pressure wound therapy (NPWT). This was an attempt to grow granulation tissue and for exudate management, in the upper and lower side, hydrofoam was used to prevent trauma.
The NPWT was installed with a continuous suction pressure of 120mmHg and was to be installed for 12 days. After five days of the NPWT installation, the wound was treated and cleaned. We observed that a lot of wound fluid was collected in the canester and the granulation was present. The suture on the upper and lower wound side was remove.
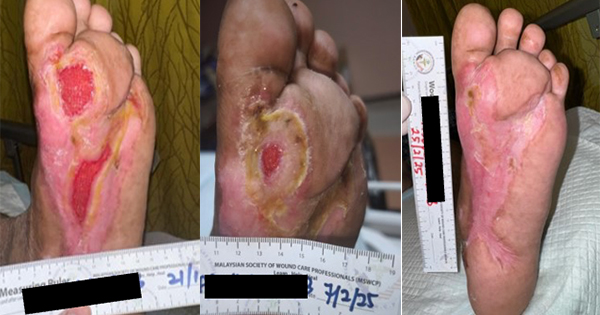
The NPWT was installed for another seven days, after which the patient was discharge to home. The next wound care treatment was in the home care setting, after the seven days NPWT, at this point wound healing was observed. The treatement had reduced the infection, the wound exudate was minimal and the epithelial cells had migrated suggesting the wound was in the remodelling phase. On day 12 the wound was cleaned by octenidine solution, then closed with a modern hydrofoam dressing to prevent epithelial trauma for the next 3 days.
Results
Implantable cardiac device SSI was effectively treated using NPWT, clearing the infection and heavy exudate volume. In this case five days NPWT with setting continuous pressure 120mmHg resulted in reduced infection, while collecting large amounts of wound fluid in container. The 2 cm hole 1cm deep in centred of wound, had granulation tissue and healing on the upper and lower side of the incision, allowing removal of the surgical suture.
Progressing to a patient home care setting, reduced the length of hospitalisation and the cost. Over the next 7 days of NPWT the wound continued to heal.
Discussion
The use of an implantable cardiac devices is indicated to save a patients life by improving the conduction function of the heart. However there is a risk of complications, including SSI. Signs of SSI caused by an implantable cardiac device include purulent wound exudate that can then be swabbed for a bacterial culture. Based on literature, infected causes of Pseudomonas aeruginosa and Staphylococcus aureus (MC Nichole, 2016). In this case, the swab of wound bed showed Staphylococus aureus bacteria. The antibiotics are determine based on the type of bacteria determined to be present.
The antibiotics treatment given during seven days in hospital, by the cardiologist were intravenously with sulbactam ampicillin 2x 1.5gr suitable with Aleka Guidelines (Hanafy et al, 2014). During wound cleansing process, infection management consisted of topical antimicrobials to destroy the bacteria. These include silver, chlorhexidine, polyhexamethylen biguinide (PHMB), Octenidine, NaOCl, Iodine with a concentration of less than 1%, Charcoal, honey and DACC for binding bacteria (Swanson et al, 2016). This present case study uses nano silver spray for fasting kill bacteria, this choice is appropriate according to the 2016 IWII guidelines.
Debridement is a technique to remove debris, necrotic tissue and reduce the bioburden (IWII, 2016). The standard in our hospital, for the complication of a pocket infection and SSI, is to remove the generator cardiac devices and treat with conventional wound care method, with insertion of sterile latex to drain the fluid from the wound. In this present case we removed the implantable cardiac device to manage the SSI and then we used suitable wound care management and advance wound care dressings.
NPWT can be used to reduce infection and heavy exudate. NPWT works by reducing oedema, lowering the risk of microbial infection and promoting tissue granulation (Apelqvist et al, 2017). Sandy-Hodgetts et al (2017) reported that using NPWT is effective for prevent surgical wound complication. According guideline WHO (2019) the prevention SSI using NPWT is a conditional recommendation with low evidence to support this indication. In this patient, with an SSI and implantable cardiac device, the problem was infection and the management of heavy exudate. Using NPWT was consistent with the literature and was effective and efficient in a home care setting once the patient was discharged from hospital.
Conclusion
SSI from an implantable cardiac device can be a complication after surgery. Preventive measures included good sterile technique. When infection occurs, a wound swab culture is necessary to determine the right type of antibiotic based on the bacteria present. Healthcare professionals must also consider using a modern dressings and moist wound healing. With large amounts of wound fluid NPWT could be the first choice to accelerate wound healing. From the description cases of SSI with an implantable cardiac device the management of heavy exudate with NPWT is effective and in our case the wound healed within 12 days.