A Caesarean section is one of the most frequently performed operations in obstetric practice. This procedure saves the lives of the mother and baby by preventing maternal and perinatal morbidity and mortality (Alfouzan et al, 2019). The decision to proceed with Caesarean section should be made carefully because the surgery has risks and complications. One of the major complications associated with Caesarean delivery is wound infection, which is one of the leading causes of morbidity and mortality, lengthening hospital stays, and affecting the mother’s ability to breastfeed, raising expenditure and increasing maternal stress (Alfouzan et al, 2019; Mhaske et al, 2020).
Methicillin-resistant Staphylococcus aureus (MRSA) is the most common organism cultured from Caesarean wound exudate (Mhaske et al, 2020). MRSA is endemic in most hospitals worldwide and it is resistant to the effects of many common antibiotics (Nandhini et al, 2022). Rational and restricted use of antibiotics are needed to control MRSA strains.
According to the Infectious Diseases Research Centre, Institute for Medical Research, Kuala Lumpur, Malaysia, the MRSA rate was 19.8% in 2017 for all medical and surgical specialities (Zainol Abdin et al, 2020). Early identification of MRSA, early diagnosis and appropriate treatment of infection is mandatory for a good prognosis (Cigaran et al, 2021).
Infection with MRSA is usually associated with worse wound outcomes. The appropriate method of wound care is essential to speed up the healing process and prevent further complications. Modern dressings serve an important role in the care of infected wounds. They regulate the humidity around the wound, which is necessary for a local defence made by macrophages to accelerate the angiogenesis process, prevent further tissue damage from over-hydration, and promote the wound healing process (Nuutila et al, 2021).
The requirements of each individual patient’s wound at any particular time need to be determined, as these may differ while a wound progresses through the healing process. Selecting the best dressings for managing infected wounds may become a challenge as many factors need to be considered.
We report a case of a wound post-Caesarean that was infected with MRSA and successfully healed using multiple modern dressings with antimicrobial properties, despite some challenges throughout the healing process.
Case presentation
A 35-year-old woman whose baby had been delivered at 37 weeks via emergency Caesarean section, presented a week after the operation with fever of 2 days’ duration that was associated with wound breakdown. Her symptoms started with being unable to walk properly after being discharged from ward on day three post operation. She experienced pain and loss of feeling in her right pelvic area. She was unable to actively lift her right lower limb for a step and needed assistance for daily activities that required her to walk.
At that time, there was no pain over the Caesarean wound. However, upon examination of the wound during a nursing home visit at 1 week post-partum, it was noted that there was serous discharge from the centre of the wound with redness of the surrounding skin. She was referred to hospital.
Examination revealed a gap of approximately 1 cm at the centre of the wound, with seropurulent discharge upon palpation. She was admitted for antibiotic therapy and scheduled for wound exploration. Her pulse rate was 76 bpm; temperature 37ºC, blood pressure 136/72 mmHg; respiration rate 20 bpm; and SPO2 98%.
Infection markers showed raised white blood cells (WBC) 11.2 × 109/l and high C-reactive protein 87.8 mg/1.
She was started on IV cefoperazone 1 g twice daily and metronidazole (500 mg once daily for a week. Wound exploration was performed in theatre the day after admission. Intraoperatively, seropurulent discharge was present in the skin layer, there was minimal pus discharge and unhealthy tissue was seen from rectus sheath layer, with a 1.5 cm gap seen over rectus sheath suture. Minimal serous discharge was also observed from rectus muscle layer. The wound was then washed and packed with gauze.
Wound inspection on day one post wound exploration noted the presence of unhealthy tissue with slough over the wound bed and subcutaneous tissue. Dressing with normal saline once daily was applied to the wound by the primary team. However, on day two of saline dressing, there was an increase in exudate, with the presence of thick biofilm and sloughy edges to the wound bed.
Results of the swab and tissue cultures taken on admission and during the exploration were traced immediately in view of the worsening wound condition. The cultures reported as surgical site infection with MRSA on swab culture and S aureus from tissue culture. Blood tests were repeated, with WBC: 7.11 × 109/1.
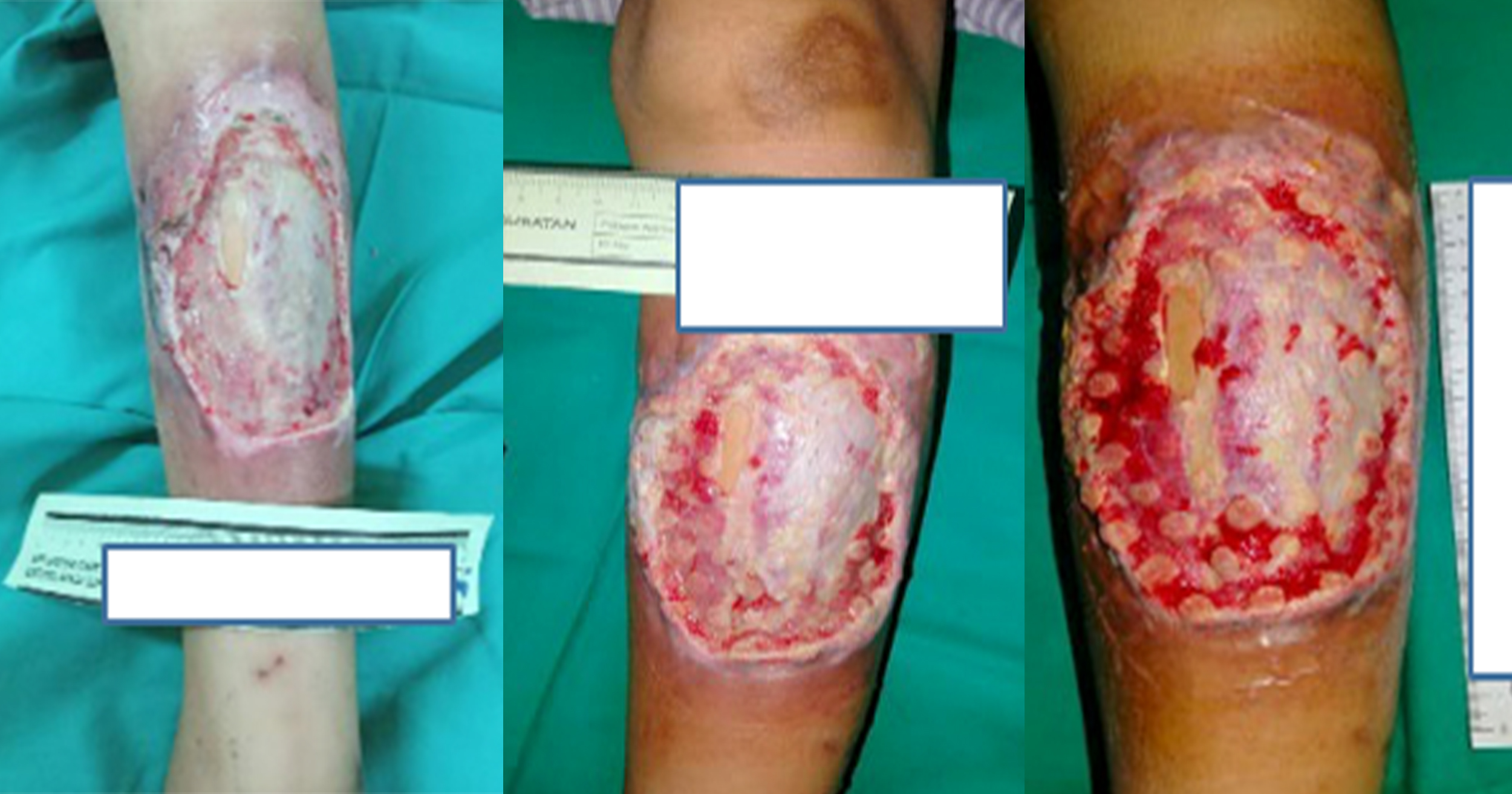
The patient was referred to the infectious disease team for escalation of antibiotic therapy and to the wound care team for wound management. Upon review by the wound team, the wound size was 16 cm × 2 cm with slough seen at subcutaneous layer, moderate exudate and thick biofilm visible [Figure 1]. HYDROCYN solution (hypochlorous acid; Bactiguard) and Nano Ag spray (a silver-containing product) were applied once daily. Then the wound bed was packed with the composite dressing AQUACEL Ag (ConvaTec), which was changed every 2 days. Significant improvement in wound healing was seen after a week on this regimen. There was less slough visible and good granulation tissue seen over subcutaneous area of the wound [Figure 2].
The patient requested that her wound dressing be performed as an outpatient in wound clinic. After reassessment of wound conditions and antibiotic requirement, this was allowed. Her wound dressing was changed to a debridement gel, Iruxol Mono (Smith + Nephew), a collagenase ointment dressing. The debridement gel was applied once daily starting from day eight post wound exploration. A clean wound with good granulation tissue was achieved after 2 weeks [Figure 3].
The primary sutures had been removed during the initial wound exploration in theatre. After 2 weeks of dressing as outpatient in wound clinic, she had achieved a clean wound with good granulation tissue and wound closure was planned. She was admitted again for secondary suturing of the whole length of the wound by obstetric and gynaecology team.
Secondary suturing of the wound was done in theatre and she was discharged the next day. Unfortunately, on day five after the secondary suturing, the wound again was complicated with a stitch abscess and there was wound breakdown at centre and edges of the wound.
She continued outpatient wound clinic follow-up with daily IODOSORB (Smith + Nephew), a cadexomer iodine powder dressing. Sutures were removed on day 14 of suturing and dressing with IODOSORB was continued once daily. Her wound progressively improved and healed well after 2 months using IODOSORB. The patient was successfully discharged from wound clinic with a healed scar 3 months post-Caesarean section [Figure 4].
Discussion
Wound care is an important aspect in the management of infected wounds. A moist wound environment is required to promote optimal healing (Nuutila et al, 2021). Numerous dressings are available for this purpose and many of these dressings have antimicrobial properties that help reduce microbial colonisation or infection.
Silver-containing dressings are modern dressings with antimicrobial properties. They are helpful in the treatment of many surgical site infections and effective against resistant organisms, such as MRSA (Thomas et al, 2011).
A study by Verbelen et al (2014) reported the effectiveness of two commonly used silver dressings, ACTICOAT (Smith + Nephew) and AQUACEL Ag, in the treatment of partial burns. The results showed that the healing time and bacterial control of the two silver dressings was similar. However, AQUACEL Ag dressings have advantages in terms of patient comfort and cost-effectiveness.
In another study, Muangman et al (2010) showed the pain-reducing function of a silver dressing (AQUACEL Ag) in patients with partial thickness burns. The results indicated that the wound healing time in the AQUACEL Ag group was significantly shorter compared with that observed in the silver sulfadiazine group. In addition, pain was also significantly reduced.
From the results in these studies, we believe that the silver dressings did benefit our patient, since frequent changes of dressing were not needed, her pain was reduced and it shortened the wound healing time.
Another modern dressing we used for our patient was Iruxol Mono, an enzymatic debridement gel. An enzymatic debrider functions as an alternative to surgical debridement, and is frequently used to achieve multiple objectives at the same time, such as infection control, pain relief, and exudate management. Collagenase is the active component of Iruxol Mono has been shown to be safe in wounds with high bacterial burdens (Payne et al, 2008).
A study by Milne et al (2010) reported a greater reduction of non-viable tissue and faster reduction in overall wound size in collagenase treated patients. The study compared collagenase and hydrogel for initial debridement of 68 patients with pressure ulcers who were treated with daily dressing followed by application of either collagenase ointment or hydrogel. Evaluation of the wound weekly for debridement progress showed 85% of the patients in the collagenase treatment arm experienced complete debridement within 42 days, whereas only 29% of those treated with hydrogel achieved complete debridement.
In our case, surgical desloughing was a challenge because most of the slough was located over the loosened stitches of the rectus muscle. Enzymatic debridement with Iruxol Mono was a good choice to debride the slough at the base of the wound. As a result, good granulation tissue and marked reduction in her wound size was seen.
Another modern dressing with antimicrobial properties that was introduced to our patient was IODOSORB powder. It is a hydrophilic starch powder that contains cadexomer iodine and is suitable for granulating wounds.
Skog et al (1983) found that cadexomer iodine was more effective than standard treatment for reduction of pain, removal of pus and debris, removal of exudate, stimulation of granulation and reduction of surrounding erythema. Bacterial infection of ulcers increased or did not change during treatment with the standard therapy, whereas cadexomer iodine significantly reduced infection with S aureus, Pseudomonas aeruginosa and other pathogenic organisms.
We applied IODOSORB powder to our patient instead of gel, since it was much easier for this powder to penetrate to the base of the wound breakdown area. The areas of wound breakdown after secondary suturing were multiple and small in size, but still worrisome because of the MRSA infection. Fortunately, her wound showed good progress and healed well with the IODOSORB powder dressing applied daily.
Our patient benefited from the regimen of modern dressings with antimicrobial properties that were applied to her wound. She experienced less emotional stress and pain, with shortened healing time, and good outcome of healing achieved. Choosing the ideal modern dressings to use has to be based on the individual wound at the time of presentation. The selection of the most appropriate dressings is still a challenge for most clinicians.
Conclusion
Modern dressings offer significant advantages in MRSA-infected wounds by inhibiting microbial activity and providing a moist environment for optimum healing. However, specific choice of modern dressings for different wounds should be individualised and based on conditions, such as the patient’s comorbidities, physiological mechanisms of the wound and the characteristics of the dressing. Selecting the dressings that are suitable for different types of wound may become a challenge to clinicians as many factors need to be considered. An appropriate understanding of individualised wound conditions and functions of each dressing available is essential to offer the best treatment to the patient.