The World Health Organisation (WHO) states that open wounds have the potential for serious bacterial infections, which may in turn lead to long-term disabilities, chronic wounds, bone infections and death. Wound infections are particularly of concern when injured patients present late for care. It is suggested that appropriate management of injuries is important to reduce the likelihood of wound infections (WHO, 2013).
Infection is a significant problem in wound management and early identification and intervention are considered critical to patients’ well-being and healing outcomes (Fletcher et al, 2021). Preventing infection mostly involves preventing wound and nosocomial infection. Wound care methods commonly include disinfection, debridement, irrigation and wound cleansing, wound drainage, local therapy, and appropriate wound closure (Ma et al, 2016).
Topical antimicrobials, including silver, differ from antibiotics, with the potential for resistance being low, and, therefore, should be regarded as an important tool in managing locally infected wounds (Kramer et al, 2018). A wide range of wound dressings containing elemental silver, or a silver-releasing compound have been developed (Ayello et al, 2012). Silver is a commonly used adjunct in wound care due to its strong antimicrobial activity (Khansa et al, 2019) and is an effective antimicrobial, which can be incorporated into an antimicrobial stewardship approach to infection management (Fletcher et al, 2021).
Silver-containing dressings are commonly considered as easy to apply, may provide sustained availability of silver, and may need less frequent dressing changes, providing potentially additional benefits, such as management of excessive exudate, maintenance of a moist wound environment, or facilitation of autolytic debridement (Ayello et al, 2012). Moreover, modern dressings have been designed to achieve better results than traditional applications such as silver sulfadiazine, which does not support optimal healing (Jiang et al, 2017) and have a tendency to form pseudo-eschar. This hinders penetration of the antimicrobial into the wound, as well as being insoluble in water, making it difficult to remove old or residual cream from the wound (Ghodekar et al, 2012).
Controversy regarding the use of silver in wound management was mainly triggered by the VULCAN Study (Michaels et al, 2009), which was considered to be a debateable study that influenced practice, notwithstanding substantial concerns that the study’s findings were theoretically misleading (Gottrup and Apelqvist, 2010; Leaper and Drake, 2011). It should be noted that, other evidence from relevant randomised studies has proven the effectiveness of silver dressings (Dissemond et al, 2017).
Clinical guidelines recommend that silver dressings should be used for the management of wounds where local infection is already established, or an excessive wound bioburden is delaying healing (Swanson et al, 2022). Silver dressings are suggested in the management of acute wounds, such as traumatic wounds, including burns), and chronic wounds that present with clinical signs of infection (Fletcher et al, 2021).
Technology Lipido-Colloid with silver (TLC-Ag)
Technology Lipido-Colloid (TLC) contains a hydrocolloid and a lipophilic substances matrix, which in vitro, has been shown to enhance proliferation of fibroblasts, as well as stimulate extracellular matrix production and contribute to the formation of new tissue through the creation of a moist environment (Bernard et al, 2005; Bernard et al, 2007; McGrath et al, 2014). TLC is also intended to reduce adhesion to both acute and chronic wound surface (Meaume et al, 2002). This atraumatic property was portrayed in an observational study that included 5,850 patients (2,914 acute wounds, 2,396 chronic wounds) (Meaume et al, 2004). Before the observational study, the wounds were being treated with traditional dressings, including gauze, paraffin-impregnated gauze, foams and hydrocolloids. When the dressings were substituted with TLC, pain reduction was reported in 88% of patients with chronic wound and in 95% of patients with acute wounds. Moreover, two non-comparative multicentre prospective clinical studies were conducted using a similar protocol in France and Germany, involving 100 paediatric patients aged 1–12 years (Letouze et al, 2004). In this trial, the dressing was also reported to be easy to apply and remove, while being conformable and nonadherent with painless removal.
In contact with the wound exudate, the silver sulphate breaks down and releases the silver ion, while the carboxymethylcellulose particles swell to form a surface hydrocolloidal film. It is suggested that this controlled supply of silver at the surface into the lipido-colloid gel guarantees a constant antibacterial activity, when the dressing is in contact with the wound (Uppal et al, 2020).
To evaluate the antimicrobial properties and the survival of a range of bacteria, including strains resistant to antibiotics of TLC-AG, in vitro analysis was carried out (White et al, 2015). The samples of dressings were inoculated with a bacterial suspension of 108 colony-forming units (CFU) and then incubated. The results showed that, from day one and throughout the duration of the study (seven days), the reduction in the number of CFUs for all the bacterial strains studied was >105 surviving bacteria. This made it possible to conclude that the TLC-Ag contact layer demonstrates antibacterial efficacy on the microorganisms tested, both Gram-positive and Gram-negative bacteria, including Pseudomonas aeruginosa, methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin resistant enterococcus (VRE) (White et al, 2015).
Furthermore, in vivo evidence includes a multicentre, phase III, controlled, randomised trial, where one cohort was treated with the TLC-Ag dressing for four weeks, followed by the neutral TLC dressing for four weeks, while the control group was treated with the neutral TLC dressing for the duration of the trial (eight weeks) (Lazareth et al, 2008). At the end of the first four weeks, the median surface area of the ulcers in the group treated with silver had decreased by 4.2cm² in comparison to 1.1cm² in the control group (p=0.0223). At the end of follow-up, the median ulcer surface area of the cohort initially managed by TLC-Ag had decreased by 5.9 cm² compared to 0.8 cm² in the control group (55% of the ulcers in the TLC-Ag group decreased by 40% or more, compared to 35% in the TLC only group throughout the study period [0.051]). At the end of the eight weeks of treatment, the relative median surface area reduction of the ulcers was significantly greater in the silver group at 47.9%, versus 5.6% in the control group (p=0.036).
TLC-Ag was also studied in a recent prospective, multicentre study on 728 patients in 39 centres for a mean duration of 26±19 days, with wounds at risk of or with local infection, under real-life conditions during the COVID-19 pandemic, conducted in Germany between May 2020 and May 2021. The clinical signs of wound infection, the most rapidly diminished clinical sign being wound deterioration. Simultaneously, in terms of the healing process, 92.1% of the wounds healed or improved, 3.2% remained unchanged and 1.7% worsened (data missing for 3.0%), and an improvement of the periwound skin was reported in 65.7% of the patients.
Also, a large, prospective, multicentre, observational study was conductedin 81 centres in Germany where the authors evaluated TLC-Ag with polyacrylate fibres in 2270 patients (Dissemond et al, 2020). Their main objective was to determine, under real-life conditions, the short-term clinical impact of the dressing on the wound healing process. The patients included in the study had both acute and chronic wounds of various aetiologies that were treated with the TLC-Ag with polyacrylate fibres for a mean duration of 22±13 days.
The authors stated that all clinical signs of local infection and the diagnosed wound infections were substantially reduced by two weeks after the treatment initiation, while all wound infection parameters continued to reduce until the last visit. Additionally, clinical improvement in wound healing was reported in 98.9% of acute wounds, with a wound closure rate of 68.5%, and, in chronic wounds, a median relative wound area reduction (RWAR) of 57.4% was achieved. An improvement in healing process was noted by clinicians in 90.6% of cases and stabilisation in 6.1%, regardless of exudate level and proportion of slough and granulation tissues in the wound bed at baseline.
Furthermore, recently, case studies regarding TLC-Ag have been reported by health professionals from Asia, which include burn wounds (Uppal et al, 2020) and other acute and traumatic wounds (Yingtao et al, 2021). In view of this, the authors elected to evaluate the dressing in their clinics in Qinzhou, China, to test TLC-Ag in three of their patients in view of implementation of the dressing in their current protocols. Throughout the study period, all the parameters of wound infection continuously decreased, resulting at the final visit in a reduction by 78.9% of the prevalence of local wound infections by 72.0%.
Case 1. Diabetic foot ulcer
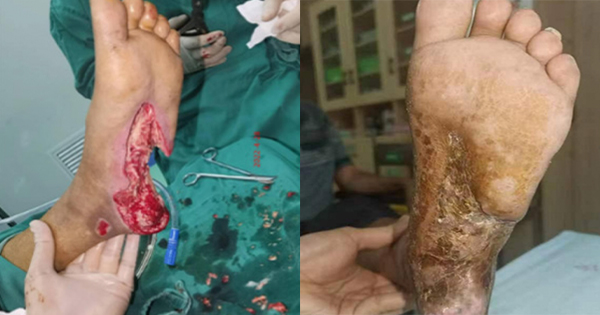
A 67-year-old male patient, with a medical history of Type 2 diabetes (diagnosed when the first foot ulcer occurred in October 2021), heart disease and hypertension, presented with an ulcer in the left plantar arch of around 12x6cm. The plantar tendon, and most of the heel bone were exposed. Necrotic tissue was present with scant exudate. It was also noted that the left dorsal artery pulse was not obvious.
Patient started complaining of ulceration in the left planta pedis that was accompanied with pain and exudation in October 2021. He was referred to Lingshan County People’s Hospital where debridement was performed and was discharged after the wound was considered as showing signs of improvement.
The patient experienced a recurrency in ulceration (four months later early February 2022) and was referred to the same clinic where another intervention was done, this time with no improvement in the wound condition. Therefore, the patient self-referred to Qinzhou First People’s Hospital outpatients, where he was admitted to the Burn Plastic Surgery (Wound Repair Department). Revascularisation of the lower limb was achieved by right femoral artery puncture for abdominal aortography and left lower extremity, arteriography and left lower extremity artery balloon dilatation (common femoral artery, superficial femoral artery, popliteal artery, peroneal artery, and posterior tibial artery).
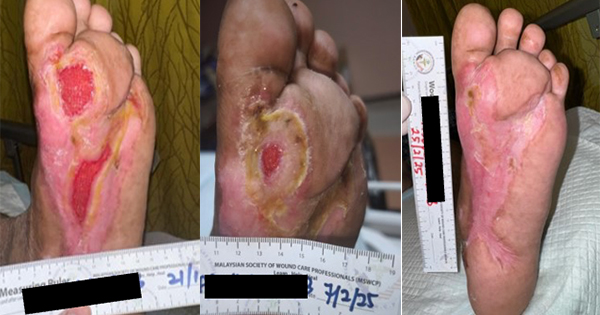
On the 28 April 2022, surgical debridement was conducted (Figure 1A), and the wound was treated with negative pressure wound therapy (NPWT) with instillation, with TLC-Ag as an interface layer (Figure 1B–C). After the operation, antibiotic, vasodilator, and anticoagulation therapy were administered with intravenous infusion. The therapy was kept in situ till 11 May.
On the 11 May, further debridement was conducted, and skin grafting applied with the TLC-Ag now applied as a primary dressing (Figure 1D–E). The dressing was changed on the 15 May (Figure 1F). Dressing changes were conducted every 2 to 3 days. Treatment continued on outpatient basis till wound closure. The wound progression is shown in Figure 1G–1I.
Case 2. Superficial partial-thickness burns/deep partial-thickness burns
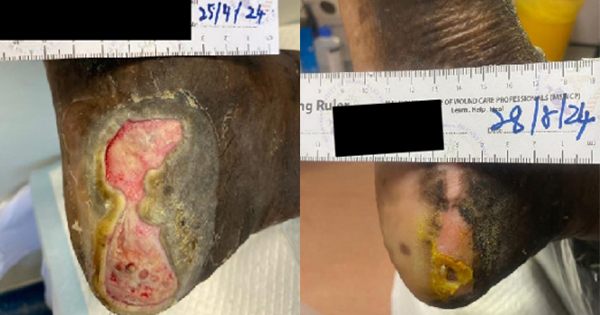
A 7-year and 4-month-old boy patient was referred after accidental gasoline flame burns of head, face, neck and right hand (superficial partial-thickness burns/deep partial-thickness burns 13%, full-thickness burns 2%). The child was admitted to Qinzhou First People’s Hospital on 15 February 2022, with severe inhalation injury (Figure 2A), requiring continuous tracheal intubation under sedation, until the airway oedema subsided on 21 February, where the tracheal catheter was removed. After extubating, the vital signs were stable.
The patient was admitted to the Hospital on 15 February 2022 (Figure 2B), and the early burn wounds were wrapped with sulfadiazine silver cream. On 28 February 2022, debridement and scab removal were performed and the dressing regime changed to TLC-AG as a primary dressing with a secondary cotton pad dressing (Figure 2C). The dressing was changed every two to three days.
On 8 March 2022, the first debridement and skin grafting were carried out under general anaesthesia (Figure 2D). The skin graft was applied with TLC-AG as an auxiliary material for back attachment, and then the wound was bandaged under positive pressure.
On 25 March, the pressure bandage was opened, and the skin graft was considered as successful (Figure 2E). Most of the skin graft pressure packs were removed, and half of the submental pressure packs was also removed.
Dressing changes with TLC-AG were continued every two to three days. The first skin graft could not completely cover the wound due to neck movement and other reasons, including that the area of skin graft is not enough to completely cover the wound, frequent neck movements which may lead to increased space between skin grafts.
Debridement and skin grafting under general anaesthesia was also conducted on the patient’s forehead on 20 March 20 (Figure 2F), the TLC-Ag was used as the primary dressing excipient and bandaging was utilised for pressure. Five days after the operation, the pressure bandage was opened, and the skin graft was successful (Figure 2G). Dressings with TLC-Ag were continued every two to three days, with maintenance debridement and wound hygiene, the wound was externally bound with the TLC-Ag dressing and cotton padding as secondary dressing. On the 30 March, all wounds were considered healed and/or healthy and the patient was discharged on 1 April.
TLC-Ag dressing changes every two to three days were continued by the Community Services. The patient was reviewed on 24 April at the clinic and the wound was considered as healed (Figure 2H).
Case 3. Repeated ulceration of penis, scrotum, and perineum
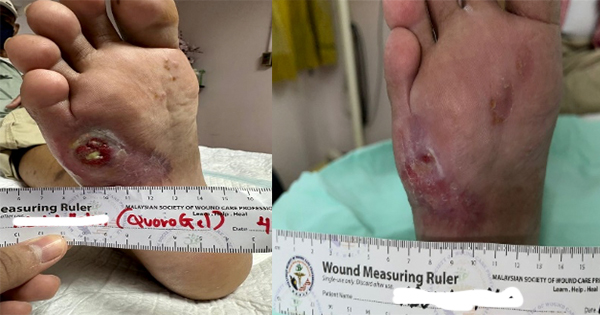
A 72-year-old male patient was complaining of repeated skin ulceration of penis, scrotum, and perineum with exudation for six months, which was ensued with ulceration, erosion, exudation, and purulent secretions. He was initially admitted on 1 September 2021, and later discharged after symptom improvement. Symptoms started recurring and the patient initiated self-medications. The condition continued to deteriorate and therefore he was re-admitted.
After consultation, he was transferred to Burn and Plastic Surgery on 10 March 2022 for the management of the ulcers (Figure 3A). He underwent perineal and scrotal ulcer debridement and excision under local infiltration anaesthesia on 16 March (Figure 3B). Further debridement and skin grafting of the perineum, penis, and scrotum as well as skin harvesting on the left thigh under spinal anaesthesia was conducted on 23 March (Figure 3C). TLC-Ag was used as a primary dressing (Figure 3D). Wound was inspected on 28 March (Figure 3E). The dressing was changed every 2–3 days. The wound was progressing, and patient was discharged on 2 April (Figure 3F). The wound healed well without changing the dressing after discharge. On 15 April 2022, few minor scars were visible but the results were very good (Figure 3G).
Conclusion
Wound dressings are essential devices in healthcare, which, according to the types and stages of wounds, can be applied to their surface to promote healing (Shi et al, 2020). Antimicrobial dressings are applied topically to the wound where they exert a broad spectrum of non-selective antibacterial action.
Misperceptions regarding the use of silver dressings were identified by a consensus group (Ayello et al, 2012), while confirming the positive effects of silver dressings when used appropriately. In vitro and in vivo evidence has shown TLC-Ag as a viable option in the management of wounds with signs of infection and others prone to infection.
The three cases from Qinzhou have shown that the use of the TLC-Ag non-adherent antimicrobial dressing, can offer health professionals an evidence-based solution to achieve better wound care outcomes. These results are concordant with those documented with TLC-Ag dressings, on patients presenting with acute and chronic wounds.