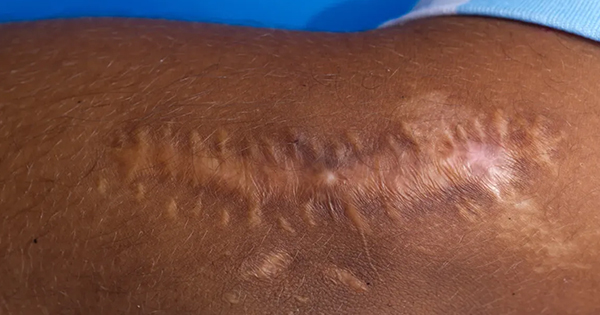
Wound healing follows a dynamic process of balanced regulation. Hypertrophic scars and keloids result from imbalances that occur in the healing process. These scars are characteristically raised as firm scars that are formed from excess fibrinogen production and collagen during healing (Berman et al, 2017). Keloids are characterised by their extensive growth which goes further than the borders of the original wound, while the edges of hypertrophic scars do not usually extend beyond the wound’s original boarders (Limandjaja et al, 2020). The process of normal healing of a wound involves inflammation, proliferation, and remodelling (Niessen et al, 1999). The initiaal phase is the most important in determining the outcome of the scar formation. Pro-inflammatory cytokines, IL-6 and IL-8, and an anti-inflammatory cytokine, IL-10, regulates inflammation. The key differences in the expression of these cytokines will lead to an increased occurrence of hypertrophic scar and keloid formation (Berman et al, 2017). Berman et al (2017) and Ferdinand et al (2019) stated that exaggeration of these inflammatory states is caused by excessive entry of inflammatory cells into the wound, which includes macrophages, lymphocytes and mast cells. These cells release cytokines including transforming growth factor β (TGF- β), which stimulates fibroblasts to synthesise the excess collagen. This abnormal scar formation results from overexpression of TGF-β1 and TGF-β2, with decreased expression of TGF-β3 leading to increased extracellular matrix (ECM) production. The scaffold of reparative tissue, the ECM, is the product of synthesis of the recruited fibroblasts. Myofibroblasts, which contain actin filaments, start the contraction of a wound. The immature scar then transitions into the final maturation phase. The abundant ECM is then degraded, and the immature type III collagen of the early wound is modified into mature type I collagen (Slemp and Kirschner, 2006). Thus, disturbance of the equilibrium between ECM protein deposition and degradation will cause an abnormal scarring appearance. Although the pathophysiology is similar, according to Brown and Bayat (2009), keloid appear as a more aggressive and sustained fibrotic disorder with a prolonged inflammatory period where immune cell infiltrate is seen, due to an increase in fibroblast activity, leading to greater and more sustained ECM deposition. This explains the characteristics of keloid scars that spread beyond the margins of the original wound. As for hypertrophic scars, it is observed that immune cell infiltrate reduces over time and stays within the original wound margins (Brown and Bayat, 2009). At a workshop conducted by Ogawa et al in 2018, a scar scale was developed known as Japan Scar Scale (JSS), that can be used as a tool to aid health professionals in differentiating between: a normal mature scar, hypertrophic scar or keloid-like lesions (Table 1). This scale will aid health professionals in grading the scar, which in turn will help choose the appropriate treatment. The classification table consists of two parts: risk factors and the present symptoms. The minimum and maximum number of points in the classification table are 0 and 25, respectively. If the classification score is 0–5, the scar fits mature scar characteristics. If the score is 6–15 or 16–25, the scar is a hypertrophic scar and a keloid, respectively. According to the scale, a patient is categorised in high-risk intractability if the lesion resembles a keloid, see for example Figure 1, thus multimodal therapy should be planned. While, if the patient is judged to be low risk intractability, the patient’s lesion resembles a hypertrophic scar (Figure 2) and conservative therapy is the primary choice (Ogawa et al, 2021). This scar scale, JSS, was applied in a retrospective cohort study by Koike et al (2014), to determine the efficacy of laser treatment for both keloid and hypertrophic scar. In the study, 102 Japanese patients with scars were classified and evaluated by using this scale before treatment was started (Koike et al, 2014). The JSS was lower in both groups (hypertrophic and keloid) after 1 year of laser treatment. However, more studies are needed to determine the accuracy of this scale in other populations and ethnic groups.
Intralesional corticosteroid injections and cryotherapy
For a keloid or hypertrophic scar that is small and recently developed (less than 12 months after wounding), the treatment of choice to date is the intralesional corticosteroid injections, that provides effective symptomatic relief via suppression of the inflammatory processes in the wound that causes pruritus. Subsequently, collagen and glycosaminoglycan synthesis reduces, fibroblast growth is inhibited, and collagen and fibroblast degeneration is enhanced. The triamcinolone acetonide (TAC) (10–40mg/mL) is generally sufficient to be given every 3–4 weeks, but in certain cases it may occasionally be continued for six months or more (Gauglitz, 2013). According to Robles and Berg in 2007, the rate of response to this method of treatment varies from 50% to 100% and rate of recurrence varied from 9% up to 50%. As for older hypertrophic scars and larger keloids, a better outcome can be achieved by combining cryotherapy with intralesional corticosteroid. Cryotherapy works by inducing vascular damage, which leads to tissue anoxia, and subsequently necrosis. For postoperative healing, a delay of approximately 3–4 weeks between sessions may be required. The author suggests that cryotherapy is conducted before the injections, as the formation of oedema induced by cryotherapy will create a larger surface area for corticosteroid to be injected, thus larger amounts can be given, ensuring a higher success rate of treatment (Gauglitz, 2013).
Pressure therapy
Pressure therapy is usually conducted with the use of pressure bandages or suits, and occasionally used with transparent plastic masks or pressure buttons in specific locations. The mechanism of action is vague, but it is understood that when capillary perfusion is limited, it will result in decreased collagen synthesis, thus reducing the supply of oxygen to the scar tissue (Kelly, 2004). The amount of pressure and the duration of therapy recommended by Macintyre and Baird (2006) is based on when the scar is still active: continuous pressure of 15–40mmHg for at least 23 hours per day for more than six months can be applied. In another similar study by Candy et al (2010), for hypertrophic scars, a continuous high-pressure group of 20–25mmHg was significantly superior to the low-pressure group (10–15mmHg) in the five months intervention programme whereby participants are prescribed with pressure garments to be worn for 23 hours a day.
Though data is provided, limitations such as the inability of the garment to fit the wounded area adequately, poor adherence by the patient and undesirable side effects when in contact with the pressure garments (e.g. maceration, eczema, and odour) make this mode of treatment less effective. In addition, some areas are not appropriate for application, such as the neck or ear.
Radiotherapy
In terms of reducing recurrence of keloids, radiotherapy is primarily used as an adjunct to keloid removal via surgery. Radiation inhibits the neovascular buds and proliferating fibroblasts will result in reduced collagen production (Ragoowansi et al, 2003). It is best to initiate the radiotherapy as early as 24 to 48 hours post-keloid excision by using a total dose of 12 Gy that is divided into six to ten fractions, which is recommended to be applied on every day or every other day (Nast et al, 2012). However, radiotherapy needs to be used with caution, since wounds located in areas such as the thyroid or breast are more prone to carcinogenesis.
Laser therapy
Castro et al (1983) suggested that laser treatment with irradiation of fibroblasts with a 1060-nm neodymium:yttrium–aluminum–garnet (Nd:YAG) can potentially reduce collagen deposition in hypertrophic scars. Subsequently, while examinations of argon and CO2 lasers showed promising result in the beginning, the recurrence rate was reported as high upon follow-up with patients.
It has also been observed that scar texture, redness, size, and pliability were significantly improved with pulse dye lasers (PDLs; Nouri et al, 2003). Nouri et al (2003) demonstrated improvements in the quality and cosmetic appearance of surgical scars upon using the 585nm PDL on the day of suture removal. However, subsequent case control studies showed no difference in findings with untreated control groups upon a longer follow-up period (Allison et al, 2003). PDL demonstrated positive outcomes in the treatment of keloids in conjunction with lasers, as well as modalities, such as steroids, pressure, or silicone gel sheeting, which can be employed to improve the treatment outcome (Connell and Harland, 2000). In individuals who are prone to developing keloids, early intervention is key; however, evidence for the role of lasers in keloid prevention is scarce.
Silicone based products
In 2002, international guidelines on scar management recommended silicone gel sheeting as a first line therapy in linear hypertrophic scars, widespread burn hypertrophic scars and minor keloids (Mustoe et al, 2002).
Silicone gels have been used for prophylaxis of unpleasant scarring in areas that are consistently affected by movement, where sheeting could not conform (Stoffles et al, 2010). However, for mature hypertrophic scars and keloids, the ultimate benefit of silicone gel remains contradictory. Silicone sheets are suggested to be applied after two weeks post-wounding, for 12–24 hours a day for a duration of 12–24 weeks (Nast et al, 2012).
Conclusion
Options for the management of excessive scarring that involve merging of techniques, such as intralesional 5-fluorouracil, interferon and intralesional cryotherapy, have demonstrated successful outcomes in a well-designed trial that will expand the current spectrum of treatment. Innovatively, some options demonstrated certain importance, such as imiquimod cream, bleomycin, photodynamic therapy, and botulinum toxin A, but more data is required for these techniques to be recommended in clinical practice (Gauglitz, 2013). However, newer and more robust studies are worth exploring for the treatment of keloids and hypertrophic scars of all stages.