In 2019, the global diabetes prevalence was estimated to be 9.3% (463 million people), which is anticipated to rise to 10.2% (578 million) by 2030 and 10.9% (700 million) by 2045, and unfortunately, one in two (50.1%) people living with diabetes do not know that they have diabetes (Saeedi et al, 2019). In Vietnam, by 2030, diabetes is projected to be one of the top seven diseases leading to death and disability (Institute of Health Metrics and Evaluation, 2017) and, in the particular case of Vietnam, it has been reported that 40–73% of individuals are unaware that they have diabetes (Quang et al, 2012).
Diabetes related complications of the foot are predominant, with global estimated prevalence of 6.3% of diabetic foot ulcers (DFU) among adults with diabetes (Armstrong et al, 2017). DFUs occur in 9.1 million to 26.1 million people living with diabetes worldwide (2015 estimates), and, globally, 12.9 million to 49.0 million persons have a history of DFU (Armstrong et al, 2017). The burden resulting from DFU not only impacts the individuals, but also their family, society, and healthcare services who experience negative influences from DFUs (Jeffcoate et al, 2018). They are unfortunately very common, result in considerable suffering, frequently recur, and are associated with high mortality rates (Jeffcoate et al, 2018). Indeed, it is estimated that every 1.2 seconds, someone in the world develops an ulcer, and, every 20 seconds someone undergoes an amputation due to diabetes (Park et al, 2023). 85% of lower-extremity amputations in persons living with diabetes are preceded by a foot ulcer (Edmonds et al, 2021). It should also be noted that around 50%–60% of DFU will develop infection, which is the leading pathology that complicates most diabetic feet (Armstrong et al, 2017), while approximately 20% of moderate or severe diabetic foot infections result in amputation at various levels (Senneville et al, 2020).
Although national statistics regarding DFU in Vietnam are currently not available, several studies conducted in the country have discussed issues related to diabetes and foot complications (Dao, 2012). It is stated that the most common diabetes-related complications in patients with type 2 diabetes was peripheral neuropathy (37.9%), while patients could be exposed very early in the disease to the risk of diabetes related ulceration, (Dao, 2012). Statistics from a particular hospital in Ho Chi Minh City reported the rate of amputation as high, in fact up to 50% (Tan et al, 2023). In this study, delayed ulcer detection and late referral, inadequate management, delayed hospitalisation for primary care, referral with severe infection, and excessive foot tissue damage, were considered as the main reasons for amputations.
Devitalised tissue and diabetic foot infection
Diabetic foot complications comprise of DFUs and diabetic foot infections (van Netten, 2022). The spread of infection to soft tissue and bone is a main contributor leading to minor and major lower-limb amputation, meaning that early diagnosis and apposite management is of utmost importance (Richard et al, 2011). Infection is defined as:
‘A pathological state caused by invasion and multiplication of microorganisms in host tissues accompanied by tissue destruction and/or a host inflammatory response’ (van Netten, 2022).
It has been stated that, considering that all chronic wounds are colonised by microorganisms, the diagnosis of a diabetic foot infection should not be based on the microbiological analysis of a wound culture but, more importantly, on clinical findings (Richard et al, 2011). Most diabetic foot infections present superficially; however, these tend to progress, sometimes rapidly, with microorganisms migrating to the subcutaneous tissues, including fascia, tendons, muscles, joints, and bones (Schaper et al, 2023). The International Working Group on the Diabetic Foot (IWGDF) and the Infectious Diseases Society of America (IDSA) classify diabetic foot infection as mild, moderate, or severe (Schaper et al, 2023). Mild is described as the presence of ≥2 manifestations of inflammation (purulence, or erythema, tenderness, warmth, or induration), but any cellulitis/erythema extends ≤2cm around the ulcer, and infection is limited to the skin or superficial subcutaneous tissues; no other local complications or systemic illness. Moderate is considered as ‘infection in a patient who is systemically well and metabolically stable but which has ≥1 of the following characteristics: cellulitis extending >2cm, lymphangitic streaking, spread beneath the superficial fascia, deep tissue abscess, gangrene, and involvement of muscle, tendon, joint or bone’. Severe is described as ‘infection in a patient with systemic toxicity or metabolic instability, for example, fever, chills, tachycardia, hypotension, confusion, vomiting, leukocytosis, acidosis, severe hyperglycaemia, or azotemia (Schaper et al, 2023).
Additionally, the presence of a viscous adherent layer of debris is a common presentation in chronic wounds and provides the ideal environment for bacteria to multiply and can prolong the inflammatory phase (Young, 2014). Moreover, the presence of the non-viable tissue in chronic wounds, including DFUs, presents a barrier to wound healing, increases odour and exudate, prevents practitioners from assessing the extent and size of the wound and has the potential to harbour microorganisms that possibly form into biofilms (Percival and Suleman, 2015). Slough, which is quite commonly present in DFU, is generally a fibrinous mass that consists multifariously of fibrin, deoxyribonucleoprotein, leucocytes, bacteria, proteinaceous material, and serous exudate (Young, 2014). Slough may become thicker and more difficult to remove the longer it is present in a wound (Black et al, 2010). It acts variously as a retardant for healthy wound bed granulation, a barrier to viewing the depth and extent of a wound, a reservoir for pathogenic organisms and a source of malodour (Young, 2014). As slough is linked to bacterial activity and is a medium for pathogenic microorganisms, acting as a reservoir for infection that may threaten the patient’s limb, the clinician must consider the presence of slough to be clinically significant, and seek to remove it to prepare the wound for healing (Young, 2014). Therefore, devitalised tissue is removed to create a clean wound bed and promote healing (Schaper et al, 2023).
Biofilm formation plays a main role in the regression and chronicity of a wound, as host defenses and treatment options and signifcantly inhibited by biofilm, making wounds more difficult to treat (Pouget et al, 2020; Alfonso et al, 2021). Surgical/sharp or vigorous debridement to remove devitalised tissue and other contaminants from the wound bed are suggested in the management of chronic wounds (Hall et al, 2019). Effective debridement techniques may result in pain for the patient, as well as costs for health system, as well as requiring a skilled/licensed practitioners (Hall et al, 2019). Furthermore, it has been suggested that debridement does not remove all biofilm, and therefore cannot be used alone, this is one of the critical principles of wound bed preparation (Schultz et al, 2017). Microorganisms begin to secrete a protective surrounding matrix known as extracellular polymeric substance within six to 12 hours after debridement, and, without disruption, the embedded microcolonies will evolve into fully mature biofilm within two to four days (Thomas, 2017). In view of this, certain wound dressing, such as polyabsorbent fibres, have been used in effectively disrupt biofilm matrix, and therefore, enhancing the efficacy of antimicrobials, such as silver (Percival, 2018).
Polyabsorbent fibres dressing with technology lipido-colloid and silver
The evaluated dressing is an advanced wound management product that consists of cohesive poly-absorbent fibres impregnated with Technology-lipido colloid (TLC) and silver (Van Hieu et al, 2021). The effectiveness of the technology lipido-colloid with silver has been shown in in vitro studies where the antimicrobial activity is seen within the first hours, for up to seven days, with >105 reduction in colony forming units for all bacterial strains (White et al, 2021), while in a randomised control trial (RCT) it was shown that, it not only decreased the signs and symptoms of local infection, but, when used in sequential treatment with a neutral TLC dressing, it also assisted in kickstarting healing, with a superior wound area reduction that the neutral dressing alone (Lazareth et al, 2012).
It is stated that the polyabsorbent fibres dressing with technology lipido-colloid and silver has characteristics that have been shown to be effective in wound cleaning due to physically binding onto wound debris such as slough. This means that once the dressing is removed, the debris that is bound onto it, are also effectively removed (Percival, 2020). The cleaning and antimicrobial mode of action of the dressing supports its use, both for the prevention and management of a localised wound infection and, to support the strategy for progression of a chronic wound to healing (Percival, 2020). This was also discussed in an RCT, where the polyabsorbent fibres had similar efficacy and safety compared with hydro-fibres but with better de-sloughing properties (Meaume et al, 2014).
The Polyabsorbent fibres dressing with technology lipido-colloid and silver was trialed in a non-comparative prospective, clinical trial involving 37 patients with chronic ulcers, clinical signs of wound infection and more than 70% of sloughy tissue at baseline. A reduction of all clinical signs of local infection wereobseerved, wound healing progression was evident with a decrease in the wound surface area, improvement of the periwound skin, reduction of sloughy tissue and an increase of granulating tissue, over a maximum period of four weeks (Dalac et al, 2016).
Antibiofilm activity was shown in in vitro studies where the biofilm population was significantly decreased of by a log of 4.6 after 24 hours of exposure (Desroche et al, 2016). The antibiofilm activity was maintained for seven days with reduction values up to 4 log, with a reduction of methicillin-resistant Staphylococcus aureus (MRSA) biofilm superior to 99.99%. The authors concluded that the polyabsorbent fibres induce a mechanical disruption of the biofilm matrix, allowing the diffusion of silver ions which produced bactericidal activity (Desroche et al, 2016).
Interesting results were achieved from an open, prospective, multicenter, real-life study conducted in Germany involving 2270 patients presenting with exuding wounds at risk of infection, or with clinical signs of infection, for a mean duration of 22±13 days (Dissemond et al, 2020). It is worthy of note that 545 (24%) presented with DFU. In the DFU group, 10% had a diagnosed wound infection and 62% presented clinical signs of infection, most frequently malodour and oedema. The polyabsorbent fibres dressing with technology lipido-colloid and silver achieved very good healing outcomes with 18% of wound closure after three weeks of treatment, 69% of clinical improvement of healing, and a relative wound area reduction of 38.8%. The diagnosed wound infections and the clinical signs of local infection and were substantially reduced since the first assessment visit. The dressing was very well or well tolerated in all patients except one, very well or well accepted by 99% of the patients, and judged very useful or useful by 93% of the physicians.
Cases studies
The authors sought to evaluate a dressing that would assist them in their daily practice by providing effective wound management in conjunction to their existing standard of care. Although good results were already being achieved, the inclusion of a modern wound dressing in their daily practice was anticipated to further improve their outcomes in DFU management. The argument behind using advanced dressings is to improve upon precise wound characteristics (Sood et al, 2014). Nonetheless, these advanced dressings need to be assessed in high level clinical trials to justify their use in daily clinical practice (Rezvani Ghomi et al, 2019).
In view of the high levels of evidence and good results, the authors endeavored to conduct an evaluation of the polyabsorbent fibres dressing with technology lipido-colloid and silver, in conjunction with the already existing evidence-based standard of care, in the management of DFU patients, in their clinics.
The evaluations were conducted in different units (endocrinology & wound healing units) of two key, well established and respected hospitals in two main cities of Vietnam (Ho Chi Minh and Ha Noi).
Case 1
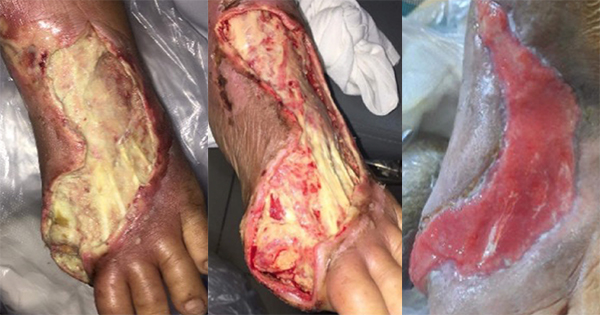
A 51-year-old female, living with hypertension and with a seven-year history of type 2 diabetes, and toe amputations, presented to the hospital on 30 May 2023 with an open ulcer on the dorsal aspect of the left foot that was present for 7 to 10 days, with exposed tendons, fully covered with slough and producing heavy exudate. The wound was initially managed with debridement, traditional dressings, and antibiotics. On the 2 June [Figure 1A], the patient was referred to the treating clinician. The wound was flushed with normal saline and polyabsorbent fibre dressing with technology lipido-colloid and silver was applied as primary dressing with a traditional secondary dressing. The dressing was changed alternate days.
By the 12 June, day 10 [Figure 1B], the slough tissue was reduced, and further improvement was seen by the 16 June, day 14 [Figure 1C]. By the 22 June, day 22 [Figure 1D], all the slough tissue was eliminated and the wound bed showed healthy granulation tissue all over and the exudate was considerably reduced.
From then, the patient was followed up in the community with the wound being managed with a protease reducing dressing (Technology Lipido-Colloid with Nano Oligo Saccharide Factor). Technology Lipido-Colloid with Nano Oligo Saccharide Factor (UrgoStart, Laboratoires URGO, France) is a healing matrix dressing that is used to decrease the levels of matrix metalloproteinases (Nair et al, 2021).
A systematic review on the dressing identified 21 different level studies involving 12,000 patients , including a double blind RCT, where the authors concluded that this dressing is an evidence-based option in the management of chronic wounds as it helps in promoting the healing environment, reduces healing times, enhances the health-related quality of life of the patients, and provides a cost-effective solution (Nair et al, 2021). It is also worth mentioning that the International Working Group on the Diabetic Foot (IWGDF) (Schaper et al, 2023), The National Institute for Health and Care Excellence (NICE, 2023) and Diabetes Feet Australia (DFA, 2021) recommend the use of this dressing in the management of DFUs.
Case 2
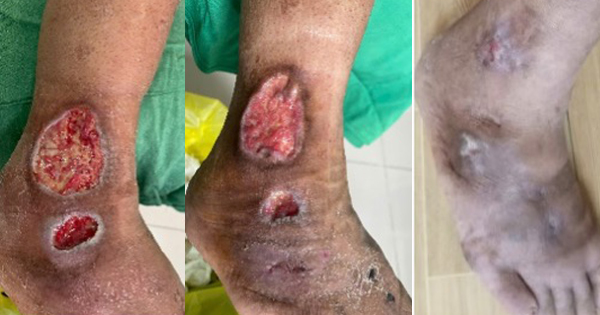
A 73-year-old female, living with hypertension and with a four-year history of type 2 diabetes, and toe amputation, presented to the hospital on 31 May 2023 with an open ulcer on the dorsal aspect of the left foot that was present for 7 days, fully covered with slough and producing heavy exudate and exposed tendons [Figure 2A]. The wound was considered at very-high risk of worsening local infection. It was flushed with normal saline and polyabsorbent fibres dressing with technology lipido-colloid and silver was applied as primary dressing with a traditional secondary dressing. The dressing was changed alternate days.
By the 8 June, day 8 [Figure 2B], the slough tissue was reduced, and further improvement was seen by the 20 June, day 20 [Figure 2C]. By the 27 June, day 27 [Figure 2D], the slough tissue was absent, and the wound bed showed healthy granulation tissue all over and the exudate was considerably reduced with further improvement and wound surface area reduction on the 1 July, day 31 [Figure 2E].
Afterwards, the patient was followed up in the community with the wound being managed with a protease reducing dressing (Technology Lipido-Colloid with Nano Oligo Saccharide Factor).
Case 3
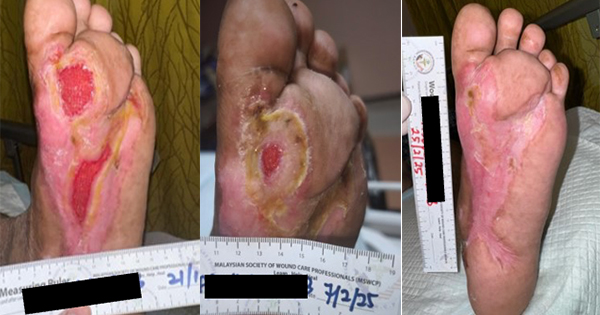
A 49-year-old female, living with hypertension and with an eight-year history of type 2 diabetes, presented to the hospital on 29 May 2023 with an open ulcer on the dorsal aspect of the left foot that was present for eight days, with exposed tendons, fully covered with slough and necrotic tissue, producing heavy exudate [Figure 3A] and considered at high risk of local infection. The wound was flushed with normal saline and the polyabsorbent fibres dressing with technology lipido-colloid and silver was applied as primary dressing with a traditional secondary dressing. The dressing was changed alternate days.
By the 1 June, day 2 [Figure 3B], the slough tissue was reduced, and further improvement was seen by the 6 June, day 6 [Figure 3C]. Toe amputation was also conducted. By the 22 June, day 22, [Figure 3D], all slough tissue was eliminated, the wound bed showed healthy granulation tissue all over and the exudate was considerably reduced with further improvement and wound surface area reduction on the 28 June, day 28 [Figure 3E].
Like the first two cases, the patient was followed up afterwards in the community with the wound being managed with a protease reducing dressing (Technology Lipido-Colloid with Nano Oligo Saccharide Factor).
Case 4
A 55-year-old male, known case of adrenal insufficiency, gout, hypertension and type 2 diabetes, presented to the hospital on the 5 July 2023 with an open ulcer on the dorsal aspect of the right foot and lateral malleolar region that was present for seven days, fully covered with slough and necrotic tissue, [Figure 4A]. The wound was flushed with normal saline the polyabsorbent fibres dressing with technology lipido-colloid and silver was applied as primary dressing with a traditional secondary dressing. The dressing was initially changed daily.
By the first dressing change on the 1st of June, day 1 [Figure 4B], the slough tissue was reduced, and the overall condition improved. Further improvement was seen by the 10th of July, day 5 [Figure 4C]. By the 16 July, day 11 [Figure 4D], the wound bed showed healthy granulation tissue. The wound was then grafted and was closed by the 30 July, day 25 [Figure 4E].
Discussion
Infection is still considered as a common problem in chronic wounds, often resulting in non-healing stagnant wounds, with significant associated patient morbidity and mortality (Siddiqui and Bernstein, 2010). About half of DFUs have clinical evidence of infection, which frequently spreads to deeper soft tissues and bone and are considered as a sentinel event (Boulton et al, 2018).
Topical antimicrobial therapy has been used on DFUs, either as a treatment for clinically infected wounds, or to prevent infection in clinically uninfected wounds, and, in this case, more wounds may heal when treated with an antimicrobial dressing than with a non-antimicrobial dressing (Dumville et al, 2017).
The authors believe that, to improve and standardise patient care outcomes, evidence-based wound management is necessary. This route will ensure timely implementation of appropriate treatment plans and clinical pathways to improve clinical outcomes and patient quality of life (Tickle, 2021).
Furthermore, the Vietnam Association of Diabetes & Endocrinology, in their 2023 guidelines for the diagnosis and treatment of diabetic foot ulcers, have recommended the use of antimicrobial dressings with antimicrobial such as the polyabsorbent fiber dressing with TLC and silver. They reiterate that this dressing is clinically effective in treating generalized infection and promotes recovery, as well as provide rapid antimicrobial and antibiofilm effects and helps cleanse wounds and maximise the effectiveness of the silver ion at the wound. Additionally, they echo the IWGDF guidelines (Schaper et al, 2023) regarding the benefits of the Technology Lipido-Colloid with Nano Oligo Saccharide Factor dressings in the management of DFU.
Through evidence-based pathways adapted by the authors, obvious benefits were manifested in these cases presented. They recommend that clinicians do use tried and tested DFU management pathways in order to provide the best solutions for patients.
Conclusion
Moving along the chronic wound towards healing involves using an appropriate dressing regime in combination with a well-established evidence-based standard of care (Britto et al, 2022).
Wound infection is still a big challenge that imposes a significant negative impact on both mental and physical health-related quality of life in individuals living with a wound (Del Core et al, 2018), including pain, odour and exudate (Totty et al, 2021), and poses increased burden on healthcare professionals and institutions (IWII, 2022).
The cases shared in this article discuss the management of four diabetes related foot ulcers managed under real-life conditions. The results show an improvement in the wounds in a relatively short period of time. These results are in line with other publications, especially the afore discussed observational study from Germany (Dissemond et al, 2020), but also resonate with other cases presented from the region (Van Hieu et al, 2021; Seshabhattaru et al, 2021).
No additional amputation was required once the risk/local infection was controlled. Following recommendations by international guidelines (DFA, 2021; Shapper et al, 2023), in three of the cases, management was continued with Technology Lipido-Colloid with Nano Oligo Saccharide Factor dressing, which has been shown to enhance wound closure of chronic wounds, and notably of DFU, with more wounds healed, more rapidly, which led to fewer risk of amputation (Nair et al, 2021) as well as providing a cost effective solution, even shown from countries from Asia (Chang et al, 2021).
Although further cases are needed to embed TLC-Ag dressings with polyabsorbant fibres as an established part of the standard of care for DFU in Vietnam, the results are very encouraging and show that this dressing can provide an effective and evidence-based solution to manage patients with wounds at risk of infection or/and with clinical signs of local infection.