The global prevalence of diabetes among the adults in 2024 was 11.1%, according to IDF Diabetes Atlas (2025), impacting 589 million adults, and is projected to increase to 13%, affecting 853 million individuals by 2050. Diabetes mellitus is a chronic metabolic disorder linked to various systemic complications. Among its most common complication is the diabetic foot ulcer (DFU), with a lifetime chance of occurrence for people with diabetes ranging from 19% to 34%. The morbidity associated with ulceration is significant, with recurrence rates of 65% within 3 to 5 years of initial healing.
Additionally, about 20% of patients with DFUs eventually undergo lower-extremity amputation, often due to non-healing wounds, severe infections, or osteomyelitis. The 5-year mortality rate following an amputation is estimated at 50% to70% (McDermott et al, 2023) exceeding mortality of most type cancers. The high recurrence rate correlates with an increased risk of wound infection, necessitating multiple hospital visits and potentially a wound debridement. This cycle results in extended treatment duration and substantially raising the healthcare expenditure for diabetes-related care. Furthermore, the impact on patients’ quality of life is profound, leading to diminished mobility, chronic pain, psychosocial distress and economic burden (Zhu et al, 2022).
At present, there are no tools available to predict the development of DFUs. Therefore, the objective of preventative management is focused on identifying the foot at-risk through a comprehensive foot examination, use of good skin care and appropriate offloading systems. When a wound occurs it should be treated using evidence-based wound care which also considers the risk of wound infections. The pathophysiology of diabetic foot ulceration involved a complex interplay between neuropathy, vascular insufficiency and the immune system. The impaired healing abilities of people with diabetes often results in a hard-to-heal wounds. Poor healing of DFUs is also linked to dysregulation of various mediators such as matrix metalloproteinases (MMPs), pro-inflammatory cytokines and growth factors (Zheng et al, 2023).
In chronic wounds, there is an elevation of MMPs that degrade exogenous EGF, along with a downregulation of extracellular matrix (ECM) components and its receptor (Barrientos et al, 2008). Conventional approaches frequently fail to address these biochemical concerns. As such, they may be inadequate in facilitating complete and sustained wound healing, particularly in cases where chronic inflammation and cellular dysfunction impede the regenerative process.
In recent years, advanced wound care has integrated therapeutic modalities involving cellular modulation aimed at regulating vital cellular function in wound healing by means of application of bioengineered skin substitutes, stem cell-based therapy, growth factors (GFs), and gene modulation therapy.
Wound healing is the process of tissue restoration following an injury. It is a sequence of biological reactions driven by haemostasis, inflammatory response, proliferation of fibroblasts and remodelling (Spielman et al, 2023). By controlling important cellular processes throughout many stages of wound healing, GFs generate great research interest in their therapeutic uses. Activated platelets release platelet-derived growth factor (PDGF) and transforming growth factor-beta (TGF-β) among the first mediators during the haemostasis phase, therefore, starting the recruitment of immune cells and fibroblasts to the wound site (Eming et al, 2014). During the inflammatory phase, PDGF encourages the chemotaxis of neutrophils and macrophages, while TGF-β and granulocyte-macrophage colony-stimulating factor (GM-CSF) regulate the inflammatory response and enhance phagocytic activity.
Various growth factors, including vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), epidermal growth factor (EGF), insulin-like growth factor-1 (IGF-1), and platelet-derived growth factor (PDGF), play a crucial role in promoting angiogenesis, granulation tissue formation, fibroblast proliferation and re-epithelialisation while in the proliferation phase (Werner and Grose, 2003; Monaghan et al, 2023). TGF-β in the remodelling phase is required for scar tissue formation, collagen synthesis and extracellular matrix (ECM) remodelling. Tissue maturation and strengthening are also influenced by connective tissue growth factor (CTGF), IGF-1, and PDGF (Falanga, 2005).
This case report highlights the progressive healing of chronic DFU in a patient treated with topical Epidermal Growth Factor (EGF) over a period of 5 weeks.
Case report
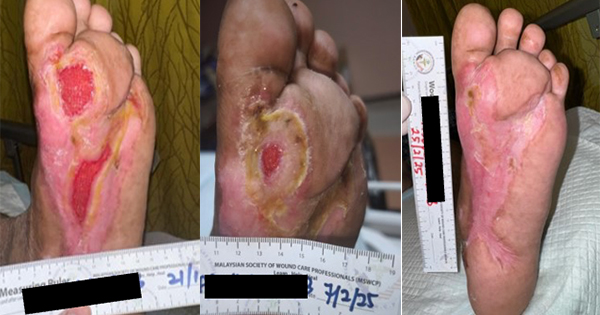
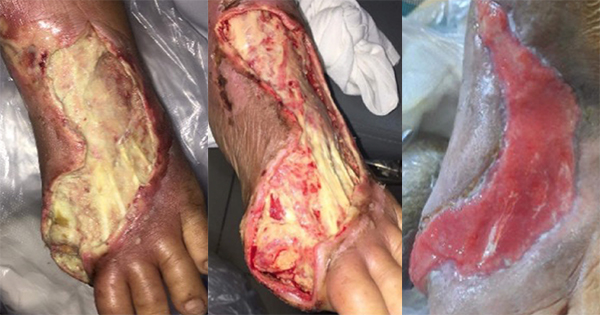
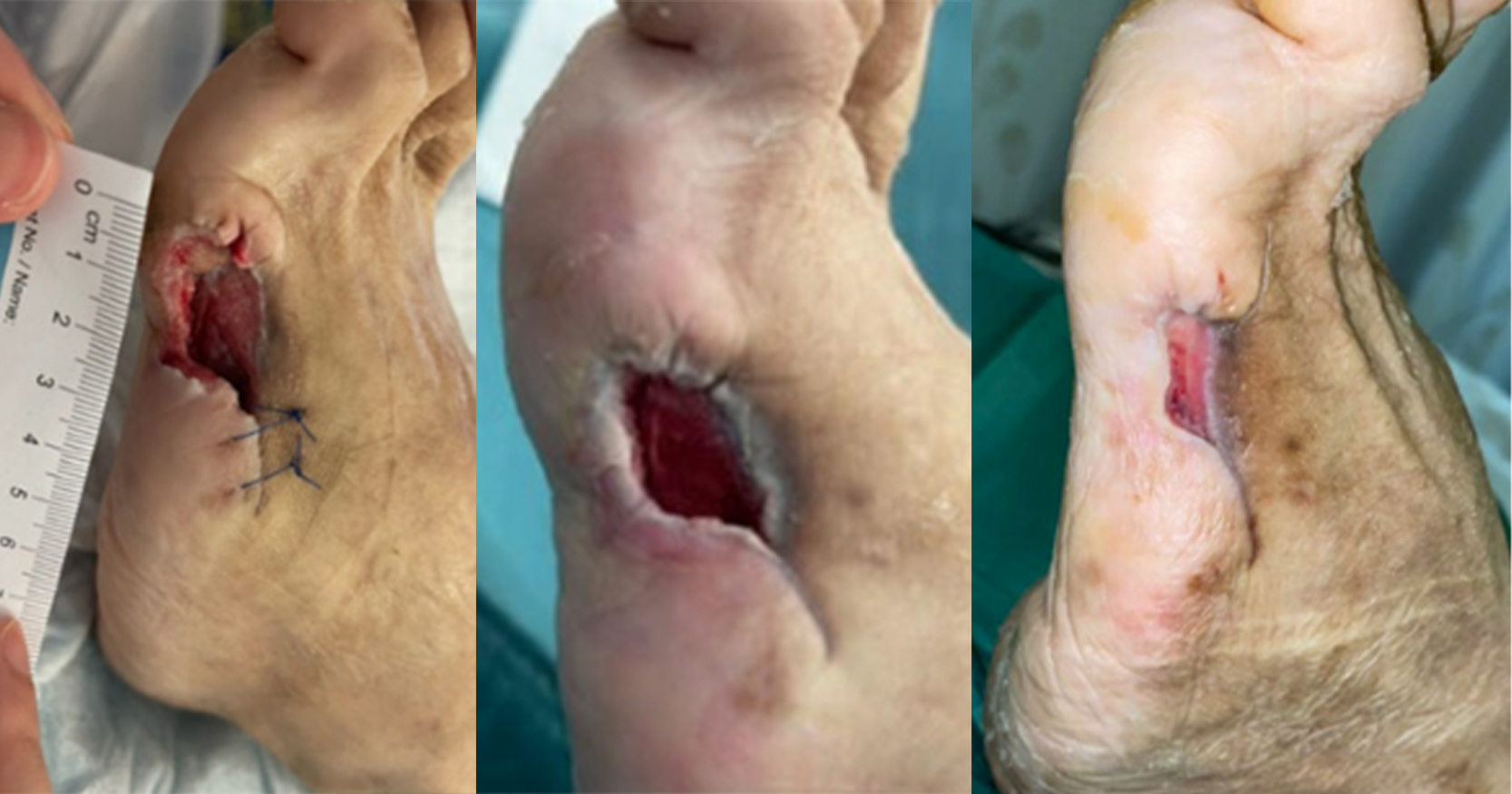
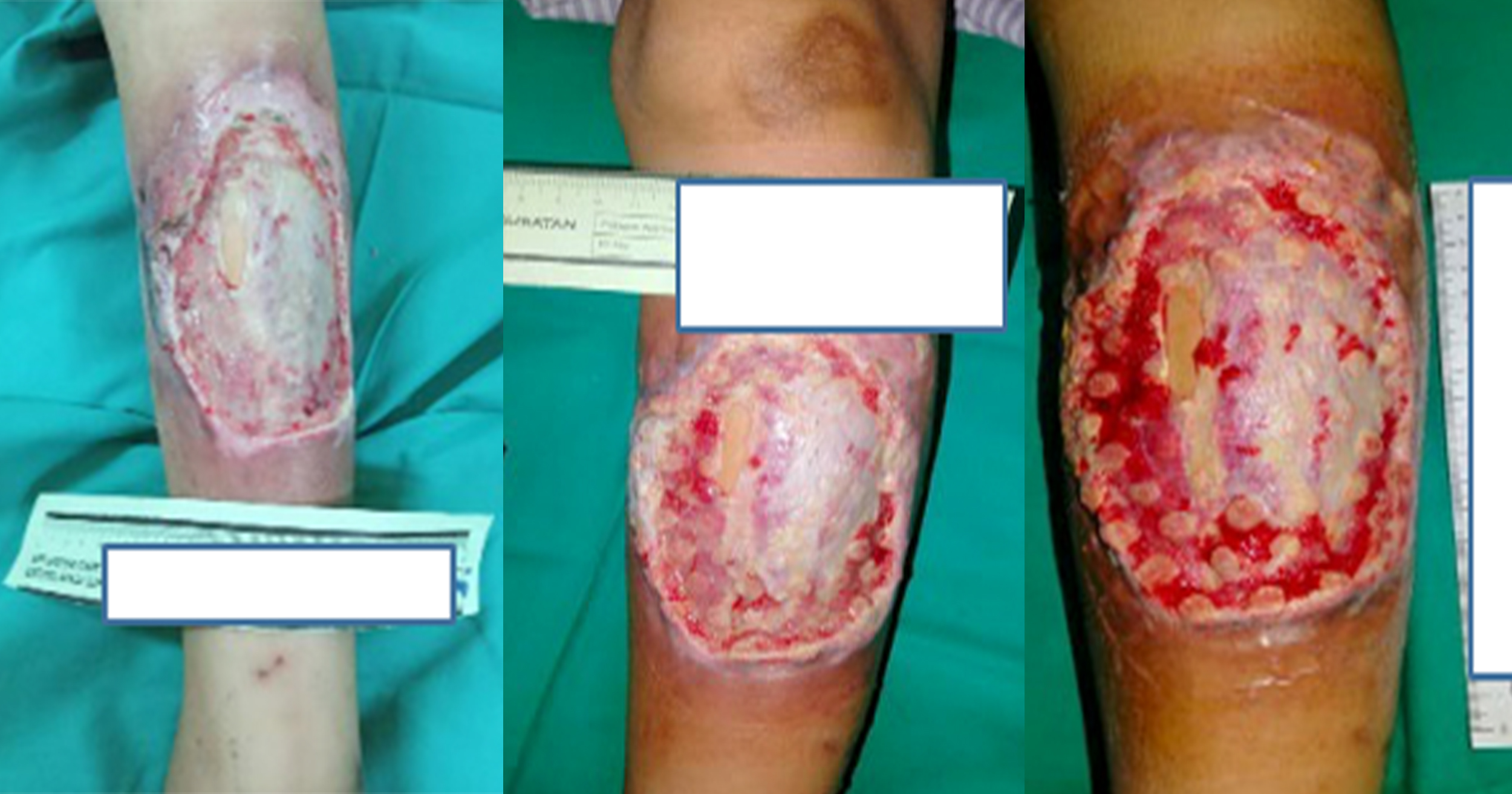
A 62-year-old male with long-standing type 2 diabetes mellitus on a basal-bolus insulin regimen presented to the wound care clinic in August 2024 after an extensive wound debridement for an infected left DFU. The patient had recurring wound infections and underwent an additional four surgical wound debridement over the course of 4 months, with the most recent procedure performed in December 2024. He had completed multiple courses of antibiotics and was being followed up by multiple departments for his diabetic foot ulcer and other comorbidities (hypertension and dyslipidaemia). His medical history is notable for peripheral neuropathy and poorly controlled blood glucose levels. On examination, there were two wounds over the plantar aspect of left foot. The wound over the metatarsal head measured 4.5cm X 4cm and the one over the midfoot measured 5cm x 4cm with good granulating base and fibrinous exudate [Figure 1]. The wound edge had callosity and surrounding scarring aligned with the history of multiple debridements. No signs or symptoms of wound infection were observed. Prior to this study, there was delayed wound healing despite use of modern dressings, offloading of the foot and adequate wound bed preparation. The overall presentation is indicative of a chronic diabetic foot ulcer in the proliferative phase that has become refractory.
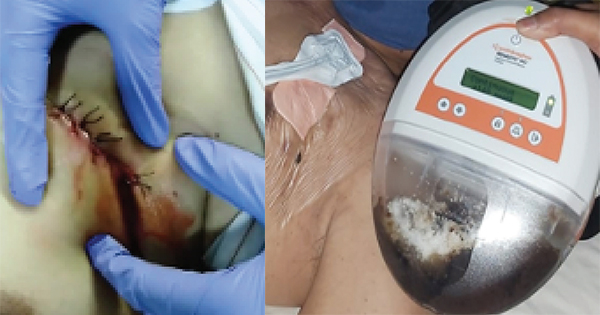
Given the chronicity of the wound and poor response to conventional therapy, topical EGF was administered as an adjuvant to promote wound healing. Standardised wound care was applied at each visit to the wound care clinic. Routine procedures included wound cleansing, debridement and callus removal from the wound edge. A single drop of topical EGF gel (Bian Skin Renewal Gel, containing EGF load of 500ppm) was then applied to each wound bed and subsequently covered with a suitable non-adherent secondary dressing based on the wound exudate. The patient was then instructed to wear customised diabetic shoe for offloading and adhere to strict sugar control at home. The patient was scheduled for a biweekly appointment in the clinic for a review and dressing change.
The patient showed rapid wound size reduction and complete healing within 5 weeks of treatment. The left midfoot wound epithelialised by week 3, while the wound over head of metatarsal achieved full closure by week 5 with healthy scar without further callus formation. The topical EGF application is well tolerated with no reported adverse effects or discomfort from the patient.
Discussion
Prior to commencing topical EGF treatment, a number of patient-related problems were experienced, that collectively caused delays in wound healing. The patient’s noncompliance with offloading footwear was one of the biggest challenges. Persistent callus formation throughout the study served as clinical evidence of repetitive pressure and friction, indicating suboptimal adherence to customised footwear.
To reduce mechanical stress, particularly on plantar foot ulcers, adequate offloading is crucial (Bus et al, 2024). Furthermore, biofilm formation is frequently associated with chronic wounds; its prevalence is as high as 78.2%, which makes the healing process more difficult (Malone et al, 2017). According to Gajula et al (2020), biofilm slows down proliferative activity and prolongs the inflammatory phase; these effects are exacerbated by the patient’s diabetes status. Poor glycaemic control is a well-recognised cause of compromised wound healing and increased susceptibility to recurrent infections. Due to a decreased cellular turnover and angiogenic response, non-modifiable factors like the patient’s advanced age inherently hinder the wound healing process (Xiao P et al, 2023).
A higher risk of developing another wound infection is highlighted in this instance by the patient’s prior numerous hospitalisations and repeated debridement for an infected DFU. If left untreated, recurrent infection could require additional surgery or even limb amputation. In order to avoid such events, a more proactive therapeutic approach is intended to speed up wound healing, with a focus on accelerating re-epithelialisation. EGF is the core component in the process in wound healing process by activation of gene expression pathways which signal the keratinocyte proliferation and migration (Blumenberg, 2013). Yang et al’s (2020) meta-analysis and systemic review of several randomised controlled trials on the effectiveness of recombinant human epidermal growth factors (rhEGF) in treating DFUs contributes support to this therapeutic strategy. In comparison to standard of care, the study showed that rhEGF significantly decreased the mean time to complete wound closure and increased the rate of wound healing. This patient achieved complete epithelialisation in 5 weeks, suggesting the possibility of even faster outcomes than the findings of Tsang et al (2003) and Hong et al (2006), which reported an average healing duration of 6 to 8 weeks. Additional data from numerous studies using topical EGF support its efficacy in treating burn wounds, chronic wounds, venous leg ulcers and DFUs (Tsang et al, 2003; Hong et al, 2006; Zaulyanov et al, 2007; Gou et al, 2020; Wei Y et al, 2022). Furthermore, applying EGF to second-degree burn wounds improves the healing process and results in lower scar scores (Zhang et al, 2024).
EGF can currently be administered in several ways, including intralesional injection directly onto the wound bed and topical application in the form of a spray, cream or gel. Because of the stability and activity in the wound microenvironment, the concentration of EGF in this formulation varies, as does its bioavailability at the wound site. Wound conditions, such as tissue hypoxia or infection can potentially induce the degradation of exogenous growth factors because of increased matrix metalloproteinase activity. The topical drug delivery may be further hampered by the presence of exudate and non-viable tissue. There is inconsistency over the ideal EGF dosage or concentration for wound healing because of the multifactorial barriers. However, Ando and Jensen’s (1993) in vitro investigation of human keratinocytes showed that EGF-induced cell migration is concentration-dependent, with the range of 10 to 50 ng/mL exhibiting the strongest stimulatory effects. This implies that in order to induce the intended biological response in wound epithelialisation, sufficient local concentration is necessary. The majority of topical formulations fall above of this range, and the most common side effect linked to topical EGF use is mild skin irritation, which usually subsides on its own with time (Tiaka et al, 2012).
This case report focusses on a single patient observation, which limits its generalisability, and the lack of a control group makes it impossible to conclusively say that topical EGF application is solely responsible for the rapid epithelialisation. Larger multicentre trials are necessary to generalise the results across a wider patient population and other wound aetiologies, even though the current study shows that topical EGF is effective in managing DFU. It is essential to further establish a standardised protocol for the proper concentration, frequency, and other administration routes of EGF application. A more comprehensive study should be done to ascertain the recurrence rate and long-term effects.
Conclusion
Topical EGF treatment demonstrated therapeutic efficacy in managing chronic wounds with significant improvements in wound re-epithelialisation, granulation tissue formation, and overall wound closure. The wound consistently decreased in size during the course of treatment, showing no signs of infection or additional complications. This clinical result demonstrates how effectively EGF worked in restoring the impaired healing that is frequently seen in diabetic foot ulcers. This case further reinforces the role of bioactive compound like EGF as essential adjuncts in management of complex, non-healing diabetic wounds.