Peripheral vascular disease (PVD) is a long-standing progressive atherosclerotic disease affecting many major arteries (e.g. the abdominal aorta, iliac arteries and lower limbs), which can potentially lead to partial or total peripheral vascular obstruction (European Stroke Organisation et al, 2011). This results in major target organ damage, which can be fatal (Gul et al, 2022). Globally, PVD has been reported with high mortalities affecting nearly 200 million people (Meijer et al, 2000; Fowkes et al, 2013). PVD has a close association with myocardial infarction, as well as stroke (Subherwal et al, 2015). On top of that, patients with cardiovascular disease and PVD have a higher risk of cardiovascular mortality when compared with those who only have cardiovascular disease. Furthermore, PVD has become a significant burden for patients as it affects their quality of life and financial wellbeing (Bevan et al, 2020).
Common risk factors of PVD include smoking, older age, diabetes mellitus, hypertension and dyslipidaemia (Meijer et al, 2000). Commonly, patients with PVD present asymptomatically, although some have symptoms such as intermittent claudication, while the most classic symptoms of PVD are paraesthesia, weakness and/or stiffness of lower limbs and cool extremities. Long standing PVD may lead to critical limb ischaemia, that presents with pain at rest, non-healing wounds and gangrene over the affected limbs (Krishna et al, 2015). The standard cost-effective and non-invasive screening method for the diagnosis of PVD is ankle-brachial index (ABI) measurement, and ABI <0.9 indicates PVD.
Dubois-Reymond has reported the presence of natural electric field in wounds in the 18th century and as wound care management strategies broaden, microcurrent therapy has become an adjunct to standard therapies. Microcurrent is an adjunct therapy in managing wound healing and providing symptomatic pain relief in PVD (Nair et al, 2018). The use of microcurrent therapy, instead of some invasive treatments, can help improve patients’ quality of life. Furthermore, it can potentially reduce the risk of lower limb amputation.
Microcurrent therapy functions by increasing the body’s production of nitric oxide and neuropeptides, increasing perfusion and reducing oedema, all of which leads to prompt relief of pain. Microcurrent therapy also resets cell signalling and electrical properties within damaged or non-healing tissues that is beneficial in acute tissue trauma, wounds, burns, scalds and fractures (Banerjee et al, 2014).
Microcurrent therapy mimics the natural electric fields created after an injury, which propagates the complex biological mechanism of wound healing (Balakatounis and Angoules, 2008). These electric fields form aids in protein redistribution directional migration, i.e. endothelial cells, keratinocytes, neutrophils, macrophages and fibroblasts; thus enhancing reepithelisation and accelerating wound healing. This biological process is known as galvanotaxis (Kloth and McCulloch, 1996).
Microcurrents are able to increase adenosine triphosphate (ATP) production radically. A sufficient amount of ATP concentration is required to accelerate wound healing. Microcurrent induction moves the electrons across the inflammatory barrier and pockets. Free radicals will be neutralised and this further stimulates cellular activity and regeneration by increasing the production of (ATP) by 500% (Cheng et al, 1982).
Microcurrents plays an important role in all phases of chronic wound healing (inflammatory phase, proliferative phase and remodelling phase). During the inflammatory phase, the electrical stimulation increases blood flow, tissue oxygenation and fibroblast stimulation (Nair and Chong, 2020), which reduces oedema and creates an antibacterial effect. Membrane transport, organisation of collagen matrix, wound contraction, DNA stimulation and protein synthesis are enhanced during the proliferative phase. The remodelling phase is important in wound closure. During this phase, microcurrent causes further fibroblast stimulation and an increase in epidermal cell proliferation, as well as migration of epidermal cells (Balakatounis and Angoules, 2008).
In addition, a study conducted in 100 patients titled “Microcurrent as an adjunct therapy in accelerating wound healing and reducing pain in patients with chronic wounds”, performed in Kuala Lumpur Hospital, has also shown the effectiveness of microcurrent therapy. Improvements were observed in parameters such as wound size, formation of healthy tissues, time taken for wound closure and degree of pain, which indicates improvement in their quality of life, as a result of improved perfusion (Nair, 2018). The study had similar findings with a two-centred retrospective study, demonstrating that the use of microcurrent has a faster and more robust wound healing process when compared with standard of care alone (Whitcomb et al, 2013).
Here we report a case study of microcurrent therapy in managing a chronic wound in a patient with a background of PVD.
Method
The patient’s caregiver was taught how to administer microcurrent therapy on the patient using an accessory electrode and adhesive pads. The carer was trained to use three modes of microcurrent therapy frequency on the patient three times a day. The modes of microcurrent therapy used in this patient are FM7-12A, SOLFEGGIO and VASO which is found in the Pro-SportTM III device (Figure 1). FM 7-12A mode transitions a combination of pre-set frequencies from 7Hz to 11Hz (Avazzia, 2023). An accessory electrode, the Y-Electrode, was placed at the patient’s left vagus nerve for two minutes on this mode, followed by another two minutes of treatment on the patient’s right vagus nerve. The [+] button on top of the device was pressed to an intensity of 20 (maximum intensity is 250) as the patient was unable to communicate if a comfortable prickling sensation was felt.
SOLFEGGIO mode was also used in treating this patient. This mode, which is named after Solfeggio frequencies, climbs from a pre-set frequency of 174Hz to 963Hz every 20 seconds. The order is then reversed and the frequencies step back down to 174Hz (Avazzia, 2023). The [+] button on top of the device was pressed to an intensity of 20 (maximum intensity is 250) as the patient was unable to communicate if a comfortable prickling sensation was felt. A Y-electrode was used to glide around the periwound skin for about 10 minutes for stimulation of the area followed by stimulation of the contra-lateral site for another 10 minutes. Subsequently, two 2cm x 2cm adhesive pads were placed on either side of the bandaged wound.
Finally, the vasodilation programme using VASO mode was selected. This mode has a pre-set fluctuating frequency between 4Hz to 99Hz (Avazzia, 2023). The [+] button on top of the device was pressed to an intensity of 20 (maximum intensity is 250) as the patient was unable to communicate if a comfortable prickling sensation was felt. The adhesive pads were left on this mode for 30 minutes.
The patient was advised to run all three modes three times a day. The patient was also advised to drink at least 500 millilitres of water after each microcurrent treatment, to eliminate waste product and toxins from the body due to lymphatic drainage.
Case report
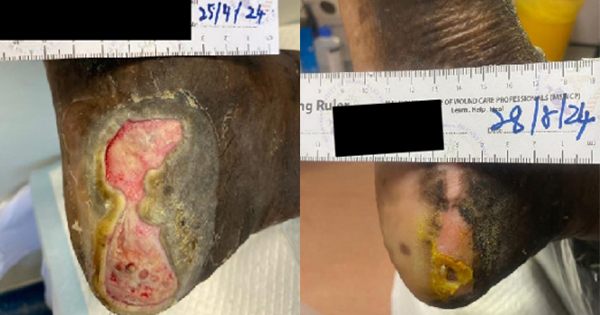
A 93-year-old Chinese lady was referred by a private general practitioner for a hard-to-heal wound over the lateral aspect of the right lower limb. She has hypertension and advanced Alzheimer’s disease. Physical examination revealed that there was an ulcer over the right lateral aspect of the shin measuring 8.0cm (length) x 6.0cm (width) x 0.2cm (depth), (Case 1A). The wound bed had slough and granulation tissue with exposed tendon (Case 1A). Ankle-brachial index (ABI) of bilateral lower limbs was found to be 0.7, raising a concern that she had compromised perfusion of the lower limbs, possibly due to PVD.
She was subsequently referred to the vascular clinic in Hospital Kuala Lumpur for further assessment for PVD. Pulses from the level of dorsalis pedis arteries, posterior tibial arteries and popliteal arteries bilaterally were diminished from Doppler ultrasound. She and her family were counselled for surgical intervention with the option of an above knee amputation by the vascular team. However, the patient and her family were not keen on amputation and opted for conservative management.
After the vascular team input was reviewed, the patient was started on microcurrent therapy, which was administered three times a day from August 2021 until February 2022 (one hour per session). The microcurrent device used was Pro-SportTM III, which features the Bio Electric Stimulation Technology (BEST; Case 1A), suitable as adjunct treatment in managing wound healing and pain control (Nair, 2018). The patient was also involved in home intervention using the Pro-Sport III model, whereby microcurrent therapy was administered at home by her caregiver.
During each dressing in the wound care clinic, the wound was assessed via TIME classification. The wound bed was described and any changes from baseline wound appearance were documented on every visit, to allow determination of changes in healing rate and wound size. Digital photographs were taken during each dressing change after wound treatment.
The patient was on standard of care treatment, which include dressing changes three times a week and microcurrent therapy administered by the caregiver three times a day. After five weeks of treatment, the wound size reduced by more than half (6.0cm [length] x 2.0cm [width] x 0.1cm [depth]), with healthy skin covering the previously exposed tendon (Case 1B). The wound was clean, with the presence of healthy granulation and epithelial tissue.
The treatment continued with standard of care treatment, with dressing changes twice a week and microcurrent therapy administered by the caregiver three times a day. After 12 weeks of treatment, the wound size further decreased to 3.0 cm (length) x 0.5cm (width) x 0.1cm (depth; Case 1C). The wound was clean with the presence of healthy granulation and epithelial tissue. After six months of treatment, the wound had healed completely (Case 1D).
Discussion
Microcurrent stimulation was used to stimulate the area around the wound site as well as to stimulate the vagus nerve. The tenth cranial nerve, also known as the vagus nerve is the longest nerve in the human body originated from the brainstem and runs through the entire body. This nerve receives and send signals to organs throughout the body by the neurotransmitter acetylcholine (Ach), which is known to play a major anti-inflammatory role in the body (Bevan and Solaru, 2020). Prolonged inflammatory processes in the body may be due to sub-optimal function of the vagus nerve. Exogenous stimulation of the tenth cranial nerve, using a low frequency of 7–11Hz, can reboot the vagus nerve and allow optimal signalling to occur (Tracey, 2015).
In this case study, delayed wound healing may be due to impaired vascular supply and old age. Microcurrent therapy was introduced on top of standard of care as a part of the treatment for the patient’s chronic wound. Pro-Sport III is a non-pharmaceutical and non-invasive device that is battery operated. The device has various modes in the form of pre-set frequency ranges and groups of pulses, which are used for a wide range of therapies. This non-invasive neurostimulation device is used for electrical nerve stimulation, improved circulation, improved range of motion, management of chronic pain, intractable pain, postoperative pain and traumatic pain (Advanced Microcurrent Solutions, 2021). The advantage of treatment with this device is that it is delivered via conductive electrode adhesive pads applied on healthy skin on either side of the bandage, thus, not having to open the wound dressing or bandage to deliver stimulation (Nair, 2018).
This therapy has been used as an adjunct method to improve wound healing, reduce pain and accelerate wound healing, the treatment improved quality of life to the patient, who may have otherwise undergone an above the knee amputation. Microcurrent therapy is proven to improve wound healing in patients by accelerating the healing process with less pain, reducing the likelihood of limb-related complications occurring (such as critical limb ischaemia or amputation), and ultimately improving patient quality of life (Foster et al, 2017).
Conclusion
Microcurrent therapy was shown to play a role in wound healing in patients with PVD, and can be used as an adjunct therapy for preventable limb amputation. Throughout the study, marked improvement was seen in the patient’s wound healing, and complete wound closure was achieved within six months. In conclusion, AVAZZIA BEST microcurrent device was able to promote wound healing.