Acute wounds follow the trajectory of four phases of wound healing, namely: hemostasis, inflammation, proliferation and maturation (Gosain et al, 2004). Wound healing may be impaired by various factors with infection being a strong extrinsic cause. Pathogenic bacteria proliferate and induce inflammatory responses that upregulate cytokines and overexpression of matrix metalloproteases (MMPs; Demidova et al, 2012). As a result, the essential extracellular matrix (ECM) and growth factors required for wound healing are broken down. Hence it is imperative to promptly detect and manage acute wound infection to prevent delayed wound healing and systemic complications such as septicemia.
Due to growing concerns about antimicrobial resistance from the indiscriminate use of systemic antibiotics, the judicious use of local antibiotics may play a role in treating localised wound infections. Topical antibiotic provides high and sustained concentrations of drugs directly in infected areas with low blood serum levels (Lipsky et al, 2009). Therefore, its application reduces the risk of systemic adverse effects and the chance of developing antimicrobial resistance. In certain cases where there is suboptimal distribution of systemic antibiotics at the infected area due to poor vascular perfusion or the existence of bacterial biofilm, the use of a local antibiotic is beneficial (Markakis et al, 2018).
Gentamicin-impregnated collagen sponges (GCS) have been used for decades in the field of surgery to prevent and treat local wound infections. The effectiveness of GCS implants in the reduction of surgical site infection is well supported by a meta-analysis by Kowalewski et al (2015) and Chang et al (2013). Gentamicin is an aminoglycoside antibiotic predominantly used against Gram-negative bacteria (Phaechamud et al, 2016). Furthermore, it exhibits bactericidal effects against prevalent Gram-positive bacteria that are responsible for wound infections, such as Staphylococcus aureus and Pseudomonas aeruginosa (Bessa et al, 2015).
Collagen is the most abundant protein in the body and part of the main component of the ECM in tissue. A natural polymer such as collagen is commonly utilised as a carrier for drug delivery systems for topical antibiotics due to its good biocompatibility, biodegradability and low antigenicity properties (Kilian et al, 2019). Collagen sponges provide scaffolds for tissue regeneration (Zhu et al, 2022), absorption of wound exudate (Chattopadhyay et al, 2014) and maintenance of a moist environment for wound healing. Furthermore, collagen-based dressings not only activate and attract immune cells and fibroblast, but are also degraded by MMPs, thus maintaining the native ECM and facilitating the healing process.
Collatamp® G, used in this case, contains type-1 purified collagen derived from bovine, with a composition of 2.8mg/cm2 of collagen and 2mg/cm2 of gentamicin sulphate. The collagen sponge is broken down through passive diffusion and by macrophages, resulting in the sustained release of gentamicin. A study found that the amount of gentamicin released locally varied from 170–9,000µg/mL within 24 hours, above the minimum inhibitory concentration at 4µg/mL required for gentamicin-susceptible organisms. Simultaneously, the serum level of gentamicin remained below 2µg/mL by 24 hours, significantly below the threshold for toxicity (Ruszczak et al, 2003).
This case report will outline the usage of GCS as a viable choice of wound dressing in the management of clinically infected lower extremity wounds.
Case report
A 77-year-old female with history of type II
diabetes mellitus on oral hypoglycaemic agents presented with right calf swelling and redness, for which she sought medical attention at a local clinic centre and was given treatment. However, her symptoms worsened with increased pain and clinical signs of spreading infection. She was then admitted to the orthopaedic department due to a right leg abscess for which she had undergone wound debridement in July 2023. No microorganisms were cultured from intraoperative pus sampling likely due to her being given antibiotics previously. Subsequently, she received a course of antibiotics and was discharged home with instructions for a daily conventional dressing change at a local clinic.
Postoperative day 11, she was referred to the wound care unit of a tertiary hospital for infected wound management. Upon review of the patient, she was clinically well with no systemic symptoms or signs of infection. The wound over the distal third of her right leg was assessed with TIME framework (T: tissue,
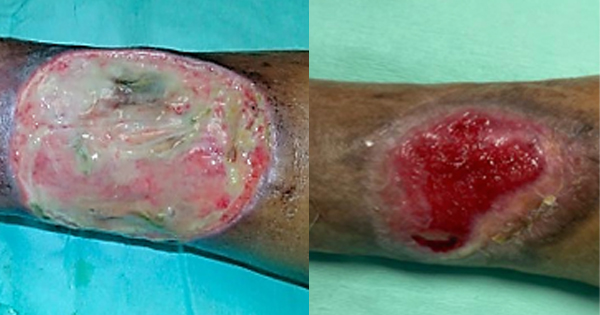
I: infection/inflammation, M: moisture balance, E: epithelial advancement) at each visit prior to treatment. The dimensions of her initial wound were recorded as 10cm x 8cm with 70% of the area covered with slough and minimal pale granulation tissue. Greenish exudate was observed on the gauze dressing and wound bed, accompanied by a moderate malodour. She also reported a Visual Analogue Scale (VAS) for pain of eight. The vascular examination revealed mild peripheral arterial disease with bilateral ankle-brachial index of 0.8. Otherwise, there were no complaints of claudication and distal pulses were well palpable.
She was given intramuscular opioid injection for the pain prior to treatment. Debridement was insufficiently carried out due to the patient’s intolerance to pain, despite analgesic. The patient’s treatment was given according to the established standard of care throughout the study period. Cleansing of wound was done with antiseptic solution along with debridement of devitalised tissue prior each dressing change. Collatamp® G (10cm x 10cm) was applied directly on the wound with light pressure to ensure it adhered well on the wound surface. Then a low-adherent absorbent dressing was used to protect and cover the GCS. The dressing was able to sustain for three days initially and later extended to a duration of five days once the exudate optimised and infection resolving.
By the eighth week of treatment, the signs of infection had completely resolved and 80% of the wound area showed good granulation. The patient also indicated a VAS score of 0 with absence of any discomfort or side effects. During the subsequent follow-up, the wound continued to epithelise and further reduction in wound size was observed without any recurrence of infection. At the 16th week, wound size reduction of 75% with healthy, viable wound bed was achieved.
Discussion
Identifying the spectrum of infection and deciding when to initiate antibiotics as therapy is a common challenge for clinicians. Not all local infections exhibit classic signs, as certain symptoms may be part of normal systemic inflammatory response to injury. In this case, the patient presented with a malodorous wound and greenish-hued exudate. Infection primarily caused by Pseudomonas aeruginosa will have more viscous and greenish exudate due to pyocyanin production by the bacteria (Cutting, 2003). Therefore, a clinical diagnosis of local wound infection was made. As per consensus by the International Wound Infection Institute, judicious use of topical antibiotics can be considered in scenarios of wound infection. The local administration of GCS resulted in the gradual release of gentamicin, reaching its highest concentration at 24 hours and sustained up to seven days. An in vitro study demonstrated the complete eradication of the Pseudomonas aeruginosa strain after 24 hours of exposure with GCS (Maczynska, 2019). Resolution of infection in this case was noted with the use of GCS alone without the need to subject the patient to further surgical debridement or systemic antibiotics.
The patient did not encounter any adverse effects during the study. No clinical manifestation of neurotoxicity or ototoxicity associated with gentamicin was reported. Wound healing is anticipated to be delayed due to the patient’s advanced old age, diabetic status and presence of peripheral arterial disease. However, there was a significant reduction of wound size of 56% by the eighth week of treatment. Randomised controlled trials evaluating the use of GCS in diabetic foot infection demonstrated higher wound healing rates compared to standard of care (Lipsky et al, 2012; Varga et al, 2014). Additionally, another study found that the formation of granulation tissue and neovascularisation are more pronounced in groups treated with GCS compared to the control group (Lee et al, 2022). However, further research should be done to identify the duration and the specific subset of patients with wound infections who would benefit from application of topical antibiotics in concordance to antimicrobial stewardship.
Limitations
This is an observational study of a single patient with limited data and selection bias, rendering it inappropriate to generalise the findings to a wider population. There are no biochemical indicators and microbiological culture to support the finding of wound infection in this case. Moreover, there are a lack of studies conducted on the usage of GCS on infected open wounds.
Conclusion
This case report concludes that the use of gentamicin-impregnated collagen sponge is effective in treating local wound infections and facilitating wound healing.