Wound healing is a complex process encompassing four distinct phases: haemostasis, inflammation, proliferation, and remodelling (Guo and DiPietro, 2010; Wang et al 2018). However, chronic or hard-to-heal wounds present a formidable challenge as they deviate from the usual healing trajectory despite appropriate treatment (Han and Ceilley, 2017)This global issue revolves around wounds that remain stuck in the inflammation phase and is particularly associated with conditions like diabetic foot ulcers (DFU), pressure ulcers (PU), and vascular ulcers, including venous and arterial ulcers (Landén et al, 2016; Zhao et al, 2016; Munro, 2017). These persistent wounds not only compromise the patient’s quality of life (QoL) but also heighten the risk of severe health consequences, such as amputations and mortality (Järbrink et al, 2017; Lo et al, 2020). Moreover, individuals suffering from chronic wounds often endure not only physical discomfort but also psychological distress, which can be exacerbated by the delayed healing process (Gouin and Kiecolt-Glaser, 2011; Hopkins, 2011). In cases where wounds produce excessive exudate, patients may experience distress and pain, compounded by concerns about dressing leakage, malodour, and staining of personal belongings like clothing, bedding etc. (Chrisman, 2010; Théorêt and Stashak, 2011).
The primary goal for healthcare providers when managing chronic wounds is to effectively address wound care, control exudate and provide pain relief. This involves the careful selection of appropriate dressing materials, as well as the implementation of adjunctive therapies tailored to specific wound types, such as offloading techniques for DFUs and compression therapy for venous leg ulcers (VLU) (Richmond et al, 2013; Bowers and Franco, 2020; Rezvani Ghomi et al 2020). Additionally, it is essential to address the patient’s underlying health conditions and factors that may impede the healing process, such as smoking, inadequate nutrition, and obesity (Han and Ceilley, 2017; The Wound Pros, 2023). Advanced therapies have emerged to address chronic wounds, such as extracellular matrices, growth factors, chitosan-based wound care dressings and collagen-based dressings. Chronic wounds that struggle to undergo complete re-epithelisation share common characteristics. These characteristics include heightened inflammation due to dysfunctional cells with limited proliferation and secretion capabilities, as well as defective mesenchymal stem cells (Briquez et al, 2015; Okurt al, 2020; Verdolino et al, 2021; Denget al, 2022). Contemporary approaches to managing chronic wounds align with the TIME framework, which encompasses tissue management, infection and inflammation control, moisture balance, and edge-of-the-wound considerations. As defined by the European Wound Management Association (EMWA), debridement refers to the removal of non-viable, necrotic tissue, biofilm, slough, scabs, and other impurities found in various types of chronic, infected, and hard-to-heal wounds (European Wound Management Association, 2004; Harries et al, 2016). Debridement is a crucial procedure aimed at preparing the wound bed to optimise subsequent treatments, ultimately leading to enhanced overall wound management outcomes. Effective debridement results in a reduction of biofilm, slough, malodour, improved microcirculation, and the stimulation of wound edges. These improvements contribute to accelerated wound healing, leading to an enhanced QoL for the patient (Leaper et al, 2012; Madhok et al, 2013; Strohal et al, 2013).
Chitosan stands as a natural biopolymer, ranking as the second most prevalent type after cellulose (Dutta et al, 2004; Aranaz et al, 2021). Chitosan is a nontoxic natural antimicrobial polymer and is approved by GRAS (Generally Recognized as Safe by the United States Food and Drug Administration)(Bellich et al, 2016; Leonida et al, 2018). This substance is extracted from chitin, primarily sourced from the shells of crustaceans, including crabs, shrimp, and lobsters (Kou et al, 2021; Iber et al, 2022). Chitosan boasts a remarkable set of attributes, including biodegradability, hemostatic capabilities, biocompatibility, anti-inflammatory properties, analgesic effects, and the ability to expedite wound closure. In the context of wound healing, Chitosan plays a pivotal role across the initial three phases of wound healing (Khan and Mujahid, 2019; Matica et al 2019; Liu et al, 2021). During the first phase, haemostasis, it actively contributes to preventing excessive bleeding by facilitating platelet and erythrocyte aggregation. The interaction between the negatively charged molecules present on the surface of activated platelets and the positively charged chitosan promotes platelet aggregation. Furthermore, this electrostatic interplay aids in the production of fibrin clots and erythrocyte aggregation. Additionally, Chitosan inhibits the production of plasminogen activators, which, in turn, safeguards fibrin breakdown and prolongs the hemostatic process (Whang et al, 2005; He et al, 2013; Pogorielov and Sikora, 2015).
During the inflammatory phase, chitosan fibres with a positive charge exhibit an affinity for and disrupt the negatively charged bacterial cell wall. This disruption leads to the release of various intracellular substances, including proteins. Additionally, chitosan penetrates the nuclei of bacteria, hindering their ability to synthesize mRNA and proteins. Chitosan and chitosan derivatives can kill microbes by neutralising negative charges on the microbial surface (Goy et al 2009; Kong et al, 2010; Atay et al, 2020). Furthermore, it helps in the conversion of pro-inflammatory macrophages (M1) to anti-inflammatory macrophages (M2) through regulating the interleukin expression level (Oliveira et al, 2012).
Thirdly, in the proliferative phase, chitosan plays a pivotal role in stimulating the formation of granulation tissue, expediting the process of skin cell proliferation. Granulation tissue comprises inflammatory cells, fibroblasts, and capillaries. Chitosan fosters the production of cytokines such as transforming growth factor-β (TGF-β), interleukin-1 (IL-1), and platelet-derived growth factor (PDGF), which are essential for macrophage activity. TGF-β, in particular, aids macrophages in homing to the injury site, promoting fibroblast growth and augmenting collagen synthesis (Kim and Kim, 2006; Periayah et al, 2014; Kiritsi et al, 2018). Consequently, this orchestrated cascade of events contributes to wound repair and skin remodelling, ultimately culminating in the complete healing of the wound.
Chitosan possesses several noteworthy characteristics, including its analgesic properties. This substance has the ability to absorb protein ions that are released at sites of inflammation, thereby reducing the production of bradykinin, gradually breaking down N-acetyl-b-D-glucosamine, decreasing the expression of the TGF-β1 factor, and inhibiting phospholipase activity. This inhibition, in turn, disrupts the arachidonic acid pathway (Okamoto et al, 2002; Harrison et al, 2020).
Maxiocel is composed entirely of grade A chitosan, derived from shellfish. One of its key advantages is its capacity to transform into a cohesive gel matrix upon contact with wound exudate. As Maxiocel transforms into this soothing gel matrix, it effectively mitigates inflammation, enhances patient comfort, safeguards the periwound area, and most importantly, expedites the wound healing process. This dressing also acts as an autolytic debridement agent, facilitating the removal of slough and necrotic tissues, thereby promoting granulation and re-epithelisation. Furthermore, Maxiocel can even remove biofilms if they adhere to the gel matrix, and the matrix is subsequently removed during dressing changes (Nair, 2022; Axio, 2023).
Method
This study is a series of outpatients cases to evaluating the clinical efficacy of Maxiocel in managing chronic, hard-to-heal wounds. Patients with chronic, hard-to-heal wounds of their lower extremities were selected, by random screening, in the Wound Care Unit, at Hospital Kuala Lumpur and were observed for up to 14 weeks. The wound was assessed and documented during each visit, this included wound size, wound bed, level of exudates, periwound skin condition, and level of pain via a validated visual-analogue scale (VAS) 1–10 between dressing changes. Table 1 lists the assessments preformed.
All patients were provided information on their treatment and were asked to sign informed consent before use of the dressing. Patients voluntarily participated and agreed that data could be used for educational purposes.
All dressings were used according to the manufacturer’s instructions. Patients’ data, medical history, surgical history, treatment, medications, allergy status, and wound assessment using the T. I. M. E. concept were documented and kept on file. Photographs were taken to monitor and record wound status and progression. Details of dressing use were also were recorded.
Inclusion criteria
Pressure ulcers
- Venous leg ulcers
- Diabetic leg ulcers
- Other leg ulcers
- Donor site wounds
- Haemostasis post debridement
- Skin abrasions
- Superficial- and partial-thickness burns
- Surgical or postoperative wound
- Able to comply with the dressing regimen at the hospital.
Exclusion criteria
- Non-compliant with the dressing regimen.
Protocol for dressing
- The previous dressings were removed from the wound
- The wound was rinsed with sterile water
- Slough and necrotic material were removed via mechanical debridement (rubbing using gauze)
- The wound is dried by dapping
- Maxiocel was applied on the wound, including approximately 1cm margin of the surrounding healthy skin.
- Melolin was applied as the secondary dressing.
- Calmoseptine cream was applied to the periwound skin.
- Gauze and gamgee were applied and the wound was bandaged.
- Wound assessment and pain score were monitored biweekly at the dressing change visit.
Results
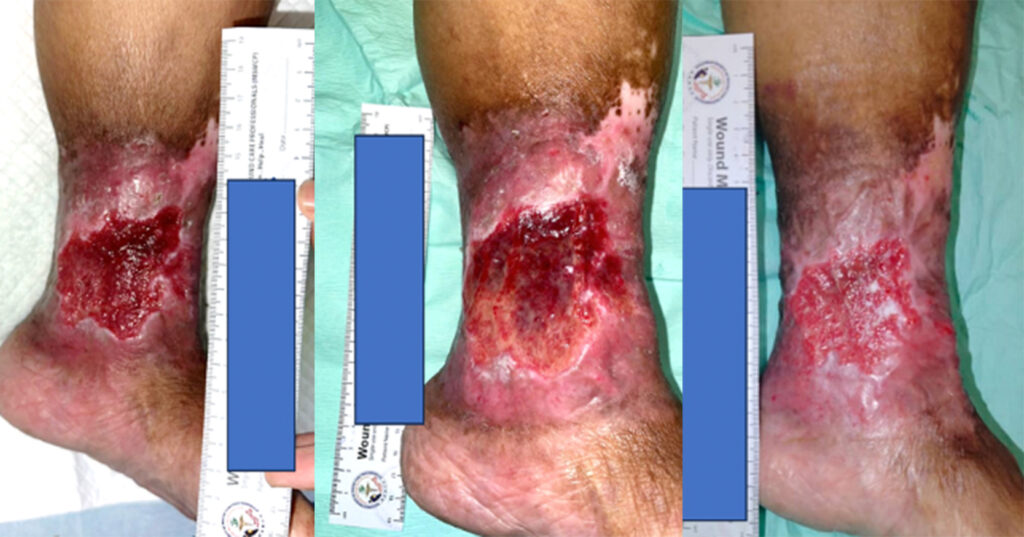
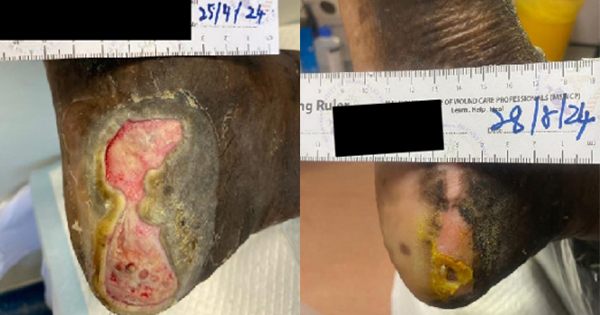
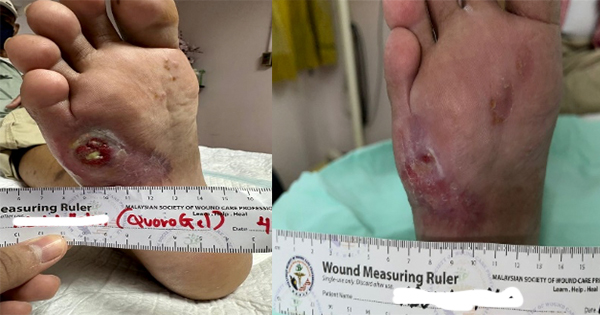
We have recruited five female patients with chronic lower limb wounds. At the start of treatment, some patient’s wounds had moderate levels of exudates and slough, which showed improvement over the course of the treatment. The wounds were dressed every 2–3 days depending on the level of exudate. The wound bed improved with a significant decrease in sloughy tissue from 10% at the start of treatment, replaced with healthy granulation and epithelial tissue. The periwound also showed improvement due to the exudate-locking capability of the dressing. The parameters assessed were wound size, wound bed appearance, periwound condition, exudate level and level of pain of the patient. All the parameters listed showed improvement within 4 weeks. The Maxiocel dressing was changed once every three days
In the 14-week evaluation, MaxioCel significantly increased the healing rate, reduced wound area, decreased slough and necrosis, and increased granulation and epithelialisation. Maxiocel also assisted with the reduction of pain as it could be removed easily in one piece. No lasting pain was reported. There was no excessive bleeding in all cases as the haemostatic properties of Maxiocel were also beneficial as it helps to control bleeding while dressing changes.
Limitations
This is a small observational study with limited number of cases. A more robust study with larger number of patients is required to confirm these preliminary results
Other limitations include the wounds assessed in this study. The cases did not include arterial ulcers. Time taken for full re-epithelisation was not considered assessed.
Conclusion
Wound bed preparation and dressing choice must provide an environment suitable to aid wound healing, protection of the wound and wound closure. To treat chronic hard-to-heal wounds, the ideal wound dressing should be chosen to maintain a suitable moisture level in the wound bed. The dressing chosen should be nontoxic, serve as a bacterial barrier, have a haemostatic characteristic for minor bleeding, and promote wound healing. Additionally, it should also manage pain and decrease scar formation at the site and should be easily removable. In this case, series MaxioCel has achieved positive outcomes through its significant healing capacities as measured by a reduction in wound surface area, improvement to the wound bed and periwound skin, and a reduction in reported pain. Chronic hard-to-heal wounds can improve and progress to healing if the bioburden is eliminated by clearing of necrotic and sloughy tissue. These objectives were achieved with the BMG dressing. The dressing has not only allowed the wound to progress toward healing but also demonstrated pain reduction in patients as it could be easily removed, in one piece.