Diabetic foot ulcers (DFU) is a serious, yet common, complication of diabetes mellitus with its global prevalence ranging from 3% to 13% (Zhang et al, 2017). The lifetime risk for any patient with diabetes of developing foot ulceration is up to 25% (Singh et al, 2005). These wounds frequently become infected, contributing to possible spread into the deeper tissue layers. Thus, surgical management of these infected DFUs may be required, as well as incision and drainage, debridement,and amputation (Armstrong and Lipsky, 2004).
The International Working Group on the Diabetic Foot (IWGDF) states that clinicians should consider negative pressure wound therapy (NPWT) as an adjunct to standard of care for the treatment of the post-surgical diabetic foot (Chen et al, 2023). NPWT has been found to promote wound granulation formation, decrease DFU healing time, and reduce wound size in contrast to conventional dressing changes (Liu et al, 2017). Armstrong and Lavery (2005) reported that a greater number of patients with partial foot amputation wounds healed and had faster healing rates with NPWT compared with standard moist wound care. There are various NPWT systems available. Some examples include ActiVAC (Kinetic Concepts Inc., KCI, an Acelity Company, San Antonio, TX, US) Invi Liberty NPWT System (Medela Inc., McHenry, IL, US) Avance NPWT System (Mölnlycke Health Care, Gothenburg, Sweden) and Renasys GO NPWT System (Smith & Nephew Inc., Fort Worth, TX, USA). These traditional NPWT systems (t-NPWT) can generate a range of negative pressures using a canister, connective tubingand a foam or gauze filler (Kirsner et al, 2019). Compared with the t-NPWT, the PICO (Smith & Nephew Inc., Fort Worth, TX, US) is a small and portable single-use NPWT (s-NPWT) system. It is battery powered, disposable, does not require a canister and delivers approximately 80mmHG of negative pressure. Furthermore, s-NPWT has been shown to be non-inferior when compared with t-NPWT systems in the treatment of chronic wounds of the lower limbs (Kirsner et al, 2019). In our current practice, t-NPWT is usually ceased once the wound is superficial and clean, rather than continuing until complete wound closure. These wounds would subsequently be treated with conventional wound care until successful healing has occurred. However, some wounds may become hard-to-heal and wound healing becomes static or delayed. Therefore, it poses potential costly implications to both the patient and healthcare system as the wounds will take a longer to heal (Dowsett et al, 2017).
This case series aims to describe two case studies in the treatment of postoperative diabetic foot wounds. They were initially treated with t-NPWT but became static hence, we transitioned to s-NPWT, which we found that it helped to stimulate the wound healing process.
Methods
A retrospective observational case series of two patients was conducted at Jurong Health Campus (Ng Teng Fong General Hospital and Jurong Community Hospital) in Singapore. This study was approved by the Domain Specific Review Board (NHG DSRB ref: 2023/00228) and informed consent was obtained. Both patients underwent lesser ray foot amputations under orthopaedic surgery and their postoperative wounds were treated by podiatrists. We used two weeks of s-NPWT to stimulate wound healing as the wounds remained static after the use of t-NPWT. The wound was cleansed with Granudacyn System (Mölnlycke Health Care, Gothenburg, Sweden) irrigation solution and bedside sharp debridement was done to remove any nonviable tissue. The periwound area was cleansed with chlorhexidine gluconate 0.05% irrigation solution before the application of s-NPWT. The s-NPWT was applied using aseptic technique. A non-sting barrier film was applied to the circumferential area around the wound, in order to prevent maceration to the periwound, before the application of s-NPWT.
Case 1
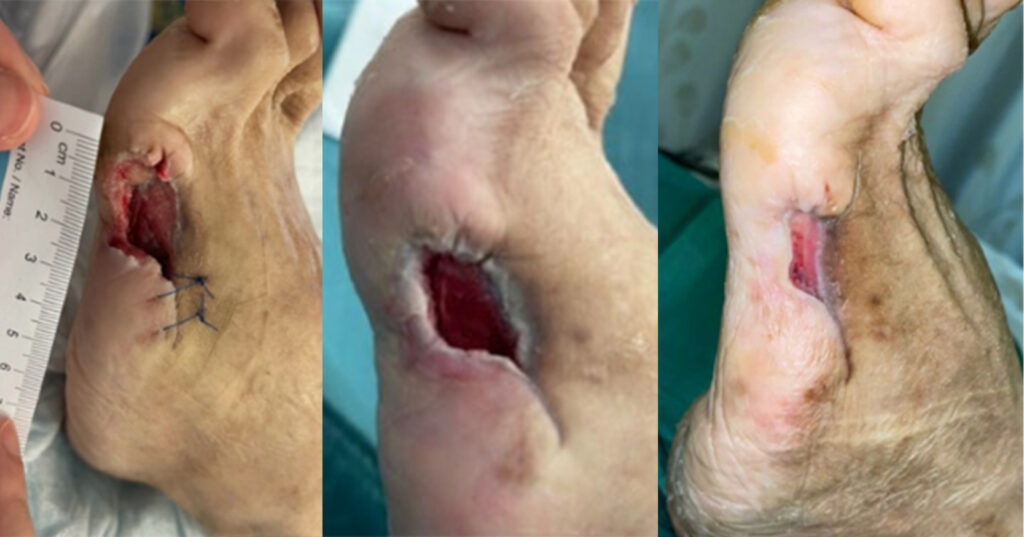
A 50-year-old woman with a history of type 2 diabetes mellitus and hyperlipidaemia was admitted into hospital due to disseminated methicillin-susceptible Staphylococcus auereus bacteraemia. Her diabetes was found to be poorly controlled as her HbA1c was 9.4% upon admission. She underwent a right below knee amputation and left fifth ray amputation for source control under orthopaedic surgery, and was also given intravenous antibiotics. Her left fifth ray amputation was initially treated with t-NPWT (ActiVAC). The NPWT system was changed every three days and the whole therapy lasted for 38 days (Case 1). Wound size at that time was 24mm x 10mm with a 6mm cavity. Subsequently, oxidised regenerated cellulose and collagen dressings (Promogran Prismamatrix, Systagenix Wound Management Limited, Gatwick, UK) and a foam secondary dressing was used, the Promogran Prisma Matrix, as it was found to have superior wound closure rates and percent wound area reduction (Chowdhry et al, 2022), and reduce protease activity levels and bacterial bioburden, which are common causes of delayed wound healing (Gottrup et al, 2013). This dressing regime was used for 26 days, with the dressing changed every three days. The wound size decreased to 20mm x 6mm, with wound surface area decreasing by 50% but the 6mm cavity was recalcitrant. The s-NPWT in combination with Promogran Prisma was subsequently used with the intention of kick-starting the healing process of the cavity. The wound was reviewed after one week and the 6mm cavity had reduced by 50% in depth. The s-NPWT was applied for an additional week. Eventually, the wound size decreased to 14mm x 2mm with the wound surface area decreasing by 76.7%. Coupled with the closure of the cavity, s-NPWT was discontinued. Conventional dressings (Promogran Prisma matrix and foam) were used, with changes done every three days. Bedside debridement was performed at every dressing change, if required. Complete wound healing was achieved 21 days (3 weeks) after the application of s-NPWT.
Case 2
A 70-year-old woman presented to the hospital with an infected DFU. (Case 2) Her medical history included type 2 diabetes, hypertension, hyperlipidaemia and peripheral arterial disease. Her diabetes was found to be poorly controlled as her HbA1c was 11.4%. She underwent a right fourth and fifth ray amputation and angioplasty. Antibiotics were also given intravenously. The amputation wound was initially treated with t-NPWT (ActiVAC; for 36 days. NPWT with instillation and dwell time; V.A.C. VERAFLO, KCI, Texas, US) was used for 17 days to aid in desloughing the wound, before switching back to ActiVAC for 10 days. The wound was treated with t-NPWT for a total of 63 days. Wound size at this point was 90mm x 12mm, with the wound base predominantly being filled with granulation tissue. However, the joint capsule and tendon of the neighbouring third metatarsophalangeal joint (MTPJ) was still exposed at the distal aspect of the wound despite undergoing almost 3 months of t-NPWT. Subsequently, s-NPWT was applied with the intention of stimulating wound healing over the third metatarsophalangeal joint. After two weeks, the wound size decreased to 75mm x 5mm with a 65.3% improvement in wound surface area and granulation tissue grew over the third metatarsophalangeal joint, thus allowing discontinuation of the s-NPWT. Conventional dressings (Promogran Prisma Matrix and foam) with dressing changes every three days were eventually used whereby the wound continued to contract in size until becoming almost fully healed by 15.7 weeks. Bedside debridement was performed at every dressing change, if required. Unfortunately, the patient passed away on due to other medical conditions before complete wound closure could be achieved.
Discussion
Overall, NPWT aids wound healing in DFUs by initiating a cascade of interrelated biological effects in the wound edge, such as stimulating contraction, and encouraging angiogenesis and the formation of granulation tissue (Malmsjö et al, 2014). Dowsett et al (2017) also suggests that s-NPWT decreases exudate levels and wound volume, while encouraging granulation tissue formation and blood perfusion, hence ameliorating the healing trajectory. While t-NPWT
relies on the transduction of negative pressure to the wound bed and edges with the use of wound fillers, no fillers were used in these two cases of s-NPWT. Malmsjö et al (2014) found that s-NPWT is able to provide similar therapeutic levels of pressure and wound contraction effects compared with t-NPWT, without the need for fillers. It is also suggestive that as the wound progresses with more granulation tissue, the fillers may potentially impede wound healing due to the physical space that they occupy (Kirsner et al, 2019). The omission of filler, alongside a non-adherent wound contact layer, can potentially diminish trauma caused to the wound tissue and periwound skin (Hurd et al, 2021). This leads to a decrease in wound bed inflammation and enhancement of healthy re-epithelialisation (Hurd et al, 2021). Hence, clinicians may consider using s-NPWT when the wound hits a certain static stage.
In addition, the two patients in this case report self-reported greater mobility and convenience with s-NPWT. They found that it was easier to participate in their rehabilitation exercises with the s-NPWT compared with the t-NPWT. They shared with the clinicians that they found the device to be more portable and easier to conceal due to its significantly smaller size. This is consistent with the findings of other studies (Dowsett et al, 2017; Hurd et al, 2021). Single-use NPWT contains a single-use pump that is battery powered, lightweight and does not require a canister as it is used on wounds with lower exudate levels (Kirsner et al, 2019). In contrast, t-NPWT is originally designed for bigger wounds with higher amounts of exudate hence the need for a canister (Hurd et al, 2021). Therefore, they restrict a patient’s ability to move freely due to its larger and bulkier design. Compared with t-NPWT, the use of s-NPWT also reduces clinician time due to its simpler application process while having similar efficacy to t-NPWT in wound healing (Kirsner et al, 2019).
In this case series, we successfully used the s-NPWT to treat diabetic foot amputation wounds after the use of t-NPWT. However, this case report did not investigate the difference of the effectiveness between the two different systems. Therefore, future studies with larger sample sizes and more robust study designs are recommended.
Conclusion
The s-NPWT system appears to be a viable treatment modality after t-NPWT in postoperative diabetic foot wounds. Clinicians may consider its usage to kick-start wound healing, especially in wounds that are found to remain static despite the use of t-NPWT. Further studies with larger sample sizes and more robust study designs may investigate the effectiveness of this treatment plan.