Chronic wounds have been described as a silent epidemic that affects a large part of the world’s population (Graves et al 2022) and some studies have defined chronic based on time to healing, with a range from 4 weeks up to more than 3 months (Mekkes et al, 2003; Mustoe et al, 2006; Leaper and Durani 2008; Werdin et al 2009; Cazander et al, 2013; Dubhashi and Sindwani, 2015; Frykberg and Banks, 2015; Martin and Nunan, 2015).
In 2021, a Consensus Panel on Chronic Wounds gathered professionals with extensive experience in clinical and research areas pertaining to wound healing (Eriksson et al, 2022). The consensus panel defined a chronic wound when standard of care has been applied with failure to progress towards healing within 4 weeks (Sheehan et al 2003; Snyderet al, 2020). If during 4 weeks of standard of care, the wound surface area is reduced by 50%, it is likely to heal on the same treatment in 12 weeks. If less than a 50% reduction occurs, it is unlikely to heal on this treatment and change of treatment should be considered (Kantor and Margolis, 2000; Sheehan et al 2003; Snyderet al, 2020). The incidence of chronic wounds is increasing both in high and low income countries in part because of the aging of the population, and the high prevalence of chronic diseases (Eriksson et al, 2022).
In the US, it is estimated that as many as 4.5 million people have chronic wounds, resulting in substantial economic and psychosocial costs. Various pathologic conditions result in chronic wound development, including arterial or venous insufficiency, diabetes, pressure ulcers (PU), presence of a foreign body, infection and others (Frykberg and Banks 2015; Joneset al, 2018).
The prevalence of chronic non-healing wounds in developed countries is 1% to 2% of the general population (Guest et al, 2015; Heyer et al, 2016), similar to the prevalence of heart failure (Roger, 2013). The morbidity and associated costs of chronic wounds, have received less attention from a public policy stand point in the US, perhaps in part because no single medical specialty has full responsibility for their treatment. Traditionally, wound care procedures to treat these wounds were done in the hospital setting (Nussbaum et al, 2018).
In 2019 there were an estimated 739 000 leg ulcers in England, with estimated healthcare costs of £3·1 billion per annum (Graves et al, 2022).
In Wales, the total cost of managing patients with chronic wounds amounted to £328.8million, an average cost of £1727 per patient and 5.5% of the total health care budget’ (Phillips et al 2016).
Diabetic foot ulcers (DFU) are common and widely acknowledged to be a source of major distress and morbidity in a predominantly elderly population, and are an enormous drain on health care resources (Reiber et al 1998; Boulton et al, 2005; Phillips et al, 2016).
It is estimated that 10% of people with diabetes will have a DFU at some point in their lives. Diabetes is also the most common cause of nontraumatic limb amputation, with diabetic foot ulcers preceding more than 80% of amputations (Graves et al, 2022).
In Brazil, Social Security data revealed that chronic venous disease is ranked 14th as a cause of temporary leave from work and occupies the 32nd position on the list of permanent disabilities. Recent studies have estimated the cost at more than US$3 billion per year. The Brazilian Institute of Geography and Statistics (IBGE) established that recurrent venous ulcer treatment was approximately US$3,479.56 per patient and that prevention measures could save nearly US$1 billion in 5 years (Camposet al, 2015; Gomes et al, 2020; Kim et al, 2021)
Direct costs
Direct costs are those related to hospitalisation or clinics, home care, outpatient treatment, emergency care, rehabilitation, examinations, medications and dressings (Hodgson and Meiners, 1982; Rice, 1999; Kirschstein, 2000; Segel, 2006; Gasparet al, 2010)
In countries like Brazil some costs are not covered by health insurance or a public health system. Sometimes, even when covered, there is a lack of products to treat wounds and medications, forcing the patient to bear high expenses or not be treated adequately.
Currently, with the advent of telehealth, artificial intelligence (AI) and smart phones, much has been done in order to and reduce expenses such as for unnecessary returns to the hospital. The possibility of monitoring patients at home can minimise travel and improve prevention of major complications, according to Prof. Chao Lung Wen, Head of the Division of Telemedicine at the School of Medicine (University of São Paulo – USP – Brazil)and President of the Brazilian Association of Telemedicine and Telehealth.
Indirect costs
They refer to the value of resources lost due to a particular disease (Kirschstein, 2000). These costs are more difficult to measure (Kirschstein, 2000; Segel, 2006). For many diseases the indirect costs can be significantly higher than the direct costs (Begley et al, 2000; Gaspar, 2010)
Intangible costs.
They refer to psychosocial costs, loss of employment opportunity, pain and discomfort, loss of quality of life (QoL), which are difficult to quantify (Gaspar, 2010).
Patients with chronic wounds have poor quality of life in general and wound-related costs are substantial. Development and implementation of wound management strategies that focus on chronic wound patients’ QoL and effectively reduce costs for this patient group are urgently needed (Olsson et al, 2019).
Skin grafts
Skin grafting is the single most effective treatment of a chronic wound. Usually, rapid coverage of the wound is preferred and autologous split-thickness skin graft represents a great option to achieve that goal. It must be always associated with optimal methods for wound bed preparation (Erikssonet al, 2022).
Mesh grafts
In 1964 Tanner described a technique for meshing and expanding split-thickness skin grafts, in order to facilitate graft take by providing drainage and making the graft more malleable. It also allowed an expansion of the graft, realistically up to 6 times. Tanner developed the Tanner-Vandeput dermatome, producing the mesh graft (It was later called ‘mesher’ and the term ‘dermatome’ became the name for the device used to procure the skin graft)
The mesher will make multiple short cuts in the graft which will create the pattern of a fish net when streched out (Tanner, 1964; Singh et al, 2017).
Minced skin grafts
Eriksson et al (2022) developed a technique for skin graft expansion with several advantages. The split-thickness skin graft was minced with a device containing parallel cutting discs spaced 0.8mm apart (Applied Tissue Technologies LLC, US). By cutting the graft in perpendicular directions, 0.8×0.8mm particles (micrografts) were created. It has been shown experimentally (Svensjö et al, 2002; Hackl et al 2012) and clinically (Danks and Lairet, 2010; Hamnerius et al, 2016) that in a moist environment, orientation of the skin particles is unimportant. With 0.8×0.8mm skin particles (micrografts) an expansion of 100 times has been achieved experimentally (Hackl et al, 2012)
The aim of this study was to compare the total direct costs of 3:1 mesh grafting (conventional treatment in the control group) with the use of the minced skin graft technique in chronic wounds.
Method
This was designed as a prospective study, with a series of 50 full-thickness chronic skin ulcers, 26 treated with MSG (Study Group MSGG) and 24 treated with 1:3 mesh grafts (control group – CG, after the Ethics committee approval (CAAE 05882918.9.0000068 – 4.991.773 / USP-HCFMUSP). All patients signed the Free Informed Consent Form. The study was conducted in accordance with the World Medical Association Declaration of Helsinki ethical guidelines.
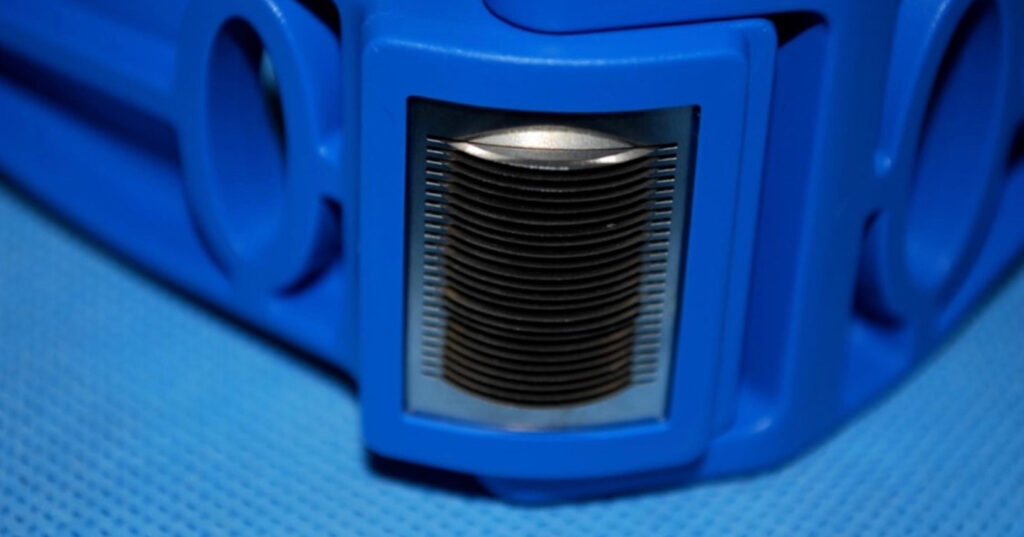
All the patients were debrided completely before entering the study. After that 26 areas were grafted with the MSG using the Xpansion® MicroAutografting Device, (Figure 1) and 24 areas were grafted with a 1:3 mesh graft (CG). Both groups were followed for 60 days. The donor site was the upper thigh, with variable sizes in the CG and a fixed size in the MSGG of 2.5cm x 5cm (12.5cm2).
Patients in the MSG study group, who underwent procedures under local anesthesia and sedation, despite being able to be discharged the same or the next day, remained hospitalised for 7 to 14 days because they did not have the minimum social conditions for home treatment and transportation for the first return visit. From a medical point of view, none of these patients would need to stay in the hospital for more than 1 day, as the dressing changes were painless and the donor areas were very small. From the socio-economic point of view, including homes without basic needs, living alone, lack of money for transportation and even to buy food, there was no possibility to release them earlier. This way we also obtained greater control of the study group as a whole. Figure 2 shows a venous leg ulcer treated with the minced skin grafts from the first procedure until complete closure, similar to sheet grafts.
Patients in the control group, however, remained hospitalised after their procedure, as is it happened routinely, waiting for the dressings to be changed in the OR, under general anesthesia, and after final graft results and donor area recovery.
It is important to remember that our public health system pays a fixed amount per day of hospitalisation according to the procedure performed, which in the case of this study was US$381.00. The number of days in the hospital from both groups were compiled in an Excel spreadsheet for statistical analysis.
Results
The median number of days in the hospital and number of days needed to stay i the hospital were 7 (interquartile range [IQR]: 7–14) and 1 (IQR: 1-1) for the MSG versus 21 (IQR: 14–30) and 18 (IQR: 14-21) for the CG, both showing statistical significance (p<0.001; Table 1).
The general mean cost of hospitalisation was US$2,939 (Standard deviation [SD]: US$1,498) in the MSG group versus US$6,477 (SD: US$2,098) in the CG (Figure 3). The average difference observed was US$3,538.00 favorable to MGF procedure, with statistical significance (p<0,001).
Dicussion
Although each country has its own health payment system, either public or private, it is well known that the treatment of chronic wounds is increasingly expensive due to the aging of the population and the large incidence of chronic diseases. In addition, many countries like ours (Brazil), lack effective programmes for the prevention of chronic diseases and lack of reimbursement of several expenses including transportation, home care, dressings and even some medications.
Our private health system, based on insurance programs and health plans, does not reimburse medications and dressings for outpatient or transportation expenses.
During the covid pandemic we improved telehealth and it became regulated in Brazil in 2022 (CFM RESOLUTION No. 2.314/2022) We do believe that our patients will benefit greatly, especially regarding the detection of complications and reduction of cost for instance now unnecessary returns to the hospital.
We are increasingly looking for treatments with a better cost-benefit ratio and that can allow procedures to be performed without hospitalisation, under local anesthesia or sedation and earlier discharge.
Our outpatients with chronic wounds, especially VLUs, experience long queues to be operated, sometimes more than 10 years.
We know that the clinical treatment of underlying diseases is essential and that medical guidelines must always be followed, but it is important that we remain open to new possibilities.
Surgical treatment of chronic ulcers using MSG makes it possible not only to perform the procedure under local anesthesia alone or with sedation, but also to allow discharge the patient on the same day or the day after the procedure.
As long as the patient has basic understanding of the process, good hygiene and is able to come to scheduled return visits, it is possible to close these wounds with a minimum donor site morbidity, faster, easier and saving cost.
The follow-up is simple and the dressing changes are almost painless.
Considering only the public health system, this could be a great development. During and after the pandemic, we still face great difficulty in the access to operating rooms and anesthesia teams.
When we consider the private health system, the possibility of performing split skin grafts under local anesthesia or sedation, in a day-hospital unit certainly would lead to a reduction in the final costs in addition to less risks for patients, especially the elderly that, when hospitalised for a longer period, are subject to more complications than the younger population.
In this paper we were able to verify that, if patients with chronic wounds in our hospital in Brazil were discharged the day after their procedure, we would have significant real cost savings.
The MSG procedure is easy to perform, with a short learning curve and minimal morbidity of the donor area. It’s comfortable for the patient, almost painless, and could allow us to treat more patients than we do now.
Undoubtedly, we still need to improve our prevention programmes and increase the development of telehealth to monitor our patients.
We believe that the use of more modern and easier technologies and reducing the total cost of the procedures, could greatly benefit the treatment of chronic wounds, not only in low-income countries but also in high-income ones.
Study Limitations
More studies are needed both in the public and private spheres, especially analysing not only the globalised daily cost but also the so-called micro costs, which consider all expenses per procedure and per hospitalisation. These should compare the procedures and expenses with longer or shorter hospitalisation time. It will also be necessary to get the patient’s opinion regarding what they consider to be more comfortable and what makes them feel more confident about the surgical procedures.
Conclusions
The MSG costs compared to the traditional 3:1 mesh graft is significantly lower for the treatment of chronic wounds.
More cost-effectiveness of interventions such as MSG technique, with full reimbursement programmes and more prevention efforts are very important not only in low-income countries such as Brazil but in high-income countries, too. It is important not only to reduce the chronic wounds patient’s financial costs but also to improve their QoL.
Treating patients with chronic wounds on an outpatient basis, under local anesthesia or sedation with minimal donor site morbidity is extremely promising. Though the future may hold pixel grafts (0.3×0.3mm), 3D printing and more, we should be using the MSG procedure more and at the same time save cost.
Cost data is required to support the choice of products and procedures. For these reasons, we encourage more studies of the costs involved in the treatment of chronic wounds to help health professionals seeking the best practices at the lowest cost.